Method for improving N-(4-methoxyphenyl)-4-acetyl-3-(1-hydroxyl)ethylazetidinone-2 synthesis conversion rate
A technology of methoxybenzene and heterocyclobutanone, which is applied in the field of medicine and chemical industry, can solve the problems of production accidents, difficulty in wastewater treatment, large amount of catalyst, etc., and achieve small solid waste generation, clear layered interface, and rapid reaction The effect of shortening the time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
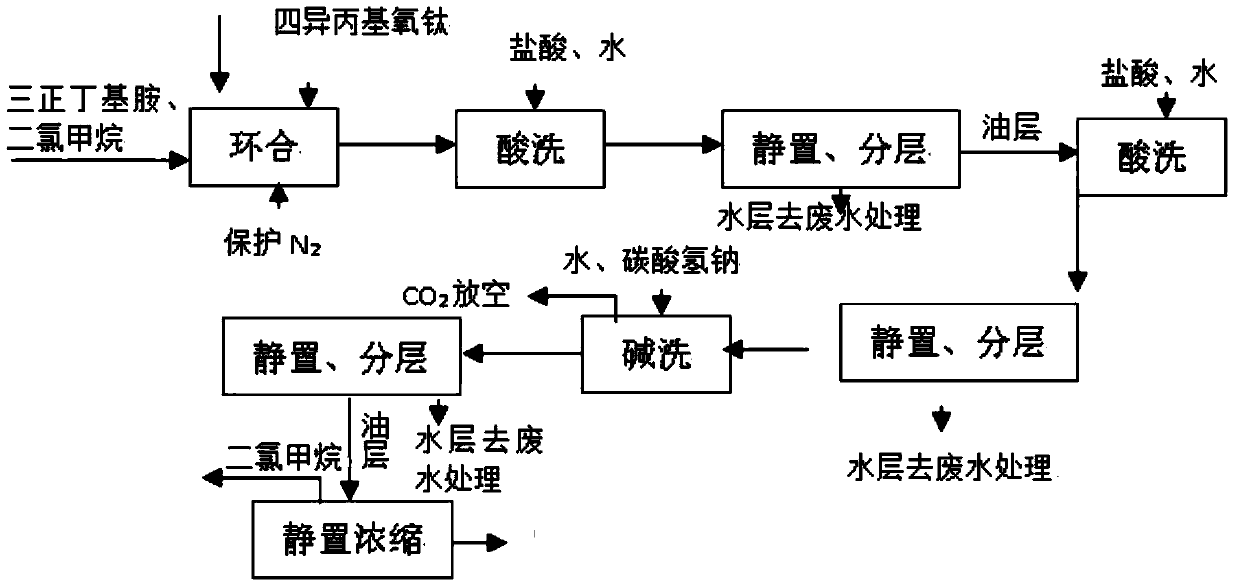
[0017] Under the protection of nitrogen, add tri-n-butylamine and dichloromethane in turn, control the temperature at about -30°C, add 220g of tetraisopropyloxytitanium dropwise, and dropwise add step-by-step at -30±5°C The obtained amide solution (containing about 0.75 mol of acylate) was added dropwise and kept at -30±5° C. for 5 hours. The reaction solution was added with 31% hydrochloric acid in an equimolar amount to the amide, and the dropping temperature was controlled at -5° C. Stir the feed solution for 30 minutes, let it stand, and separate layers. The acid water layer goes to waste water treatment, and the organic layer is added with 31% hydrochloric acid in an equimolar amount to the amide for the second pickling, static layering, and the acid water goes to waste water treatment. The layer is transferred to the washing pot, then add 100g baking soda and 50g water for alkali washing, stir for 1 hour, let stand for 1 hour, separate layers, remove the water layer for w...
Embodiment 2
[0019] Under the protection of nitrogen, add tri-n-butylamine and dichloromethane in turn, control the temperature at about -30°C, add 240g of tetraisopropyloxytitanium dropwise, and after the addition is completed, add step-by-step at -30±5°C The obtained amide solution (containing about 0.75 mol of acylate) was added dropwise and kept at -30±5° C. for 5 hours. The reaction solution was added with 31% hydrochloric acid in an equimolar amount to the amide, and the dropping temperature was controlled at -5° C. Stir the feed solution for 30 minutes, let it stand, and separate layers. The acid water layer goes to waste water treatment, and the organic layer is added with 31% hydrochloric acid in an equimolar amount to the amide for the second pickling, static layering, and the acid water goes to waste water treatment. The layer is transferred to the washing pot, then add 100g baking soda and 50g water for alkali washing, stir for 1 hour, let stand for 1 hour, separate layers, remo...
Embodiment 3
[0021] Under the protection of nitrogen, add tri-n-butylamine and dichloromethane in turn, control the temperature at about -30°C, add 260g of tetraisopropyloxytitanium dropwise, after the dropwise addition, at -30±5°C, add step by step The obtained amide solution (containing about 0.75 mol of acylate) was added dropwise and kept at -30±5° C. for 5 hours. The reaction solution was added with 31% hydrochloric acid in an equimolar amount to the amide, and the dropping temperature was controlled at -5° C. Stir the feed solution for 30 minutes, let it stand, and separate layers. The acid water layer goes to waste water treatment, and the organic layer is added with 31% hydrochloric acid in an equimolar amount to the amide for the second pickling, static layering, and the acid water goes to waste water treatment. The layer is transferred to the washing pot, then add 100g baking soda and 50g water for alkali washing, stir for 1 hour, let stand for 1 hour, separate layers, remove the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com