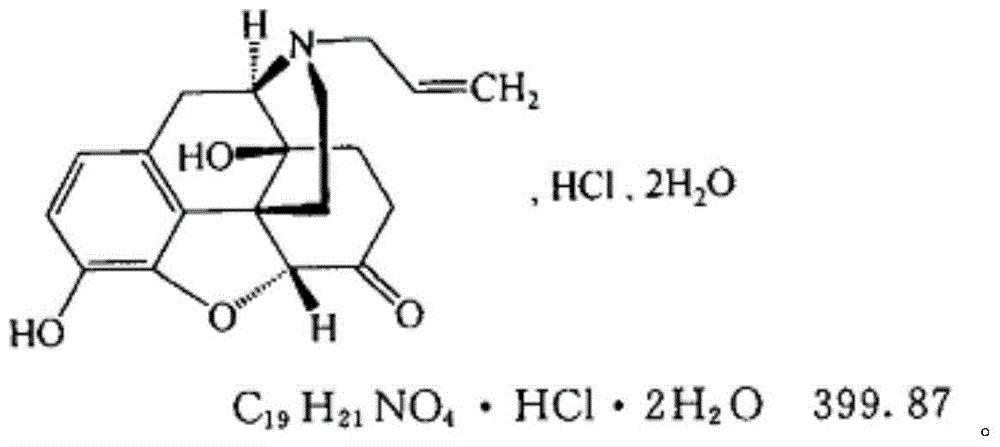
Pharmaceutical composition and preparation method of naloxone hydrochloride powder for injection
A technology of naloxone hydrochloride powder injection and naloxone hydrochloride, which is applied in the field of medicine and can solve problems such as accumulation and subcutaneous granuloma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Embodiment 1: Preparation of naloxone hydrochloride pharmaceutical composition
[0100] formula:
[0101] Naloxone Hydrochloride 1mg,
[0102]Maltose 30mg,
[0103] Citric acid 0.3mg;
[0104] Preparation method:
[0105] (a) Weighing naloxone hydrochloride, freeze-dried excipients and pharmaceutical auxiliaries of the prescribed amount, adding water for injection to dissolve, then adding activated carbon, stirring, and decarbonizing by filtration;
[0106] (b) Add water for injection to 1ml, stir evenly, measure the pH value of the solution and optionally determine the content of active ingredients, and adjust to pH 3.3-3.7 with an acid-base regulator if necessary;
[0107] (c) sterilize and filter the medicinal liquid, fill it in vials, and divide 1ml into each bottle;
[0108] (d) Freeze-drying to remove moisture, and stoppering, to obtain.
Embodiment 2
[0109] Embodiment 2: Preparation of naloxone hydrochloride pharmaceutical composition
[0110] formula:
[0111] Naloxone Hydrochloride 1mg,
[0112] Maltose 20mg,
[0113] Citric acid 0.5mg;
[0114] Preparation method:
[0115] (a) Weighing naloxone hydrochloride, freeze-dried excipients and pharmaceutical auxiliaries of the prescribed amount, adding water for injection to dissolve, then adding activated carbon, stirring, and decarbonizing by filtration;
[0116] (b) Add water for injection to 1ml, stir evenly, measure the pH value of the solution and optionally determine the content of active ingredients, and adjust to pH 3.3-3.7 with an acid-base regulator if necessary;
[0117] (c) sterilize and filter the medicinal liquid, fill it in vials, and divide 1ml into each bottle;
[0118] (d) Freeze-drying (using freeze-drying curve B) to remove moisture, and stoppering to obtain the product.
Embodiment 3
[0119] Embodiment 3: Preparation of naloxone hydrochloride pharmaceutical composition
[0120] formula:
[0121] Naloxone Hydrochloride 1mg,
[0122] Maltose 75mg,
[0123] Citric acid 0.2mg;
[0124] Preparation method:
[0125] (a) Weighing naloxone hydrochloride, freeze-dried excipients and pharmaceutical auxiliaries of the prescribed amount, adding water for injection to dissolve, then adding activated carbon, stirring, and decarbonizing by filtration;
[0126] (b) Add water for injection to 1ml, stir evenly, measure the pH value of the solution and optionally determine the content of active ingredients, and adjust to pH 3.3-3.7 with an acid-base regulator if necessary;
[0127] (c) sterilize and filter the medicinal liquid, fill it in vials, and divide 1ml into each bottle;
[0128] (d) Freeze-drying to remove moisture, and stoppering, to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com