Fluorine-perylene bisimide molecule internal-energy transferring fluorescence split compound and preparation method thereof
A fluorescent compound and perylene imide technology, applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve the problem of low two-photon absorption ability, achieve improved absorption performance, increase frequency doubling effect, and increase charge The effect of transfer degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment one: the preparation of fluorescent compound A:
[0029]
[0030] Fluorescent Compound A
[0031] Weigh 3.20g (5mmol) of 1,6,7,12-tetrachloro-N,N'-di-n-butyl-3,4,9,10-perylenediimide and 4.38g (12.5mmol) of bisphenol fluorene ) and 1.10 g of anhydrous potassium carbonate were added to a 500 mL three-necked flask, and then 350 mL of N-methylpyrrolidone was added. Under stirring with nitrogen, it was heated to 140°C and reacted for 6 hours. Remove from heat and cool to room temperature. The aforementioned reaction system was poured into 1000 mL of water, and the pH was adjusted to 7 with 1N HCl aqueous solution under stirring. A large amount of purple-red solid was precipitated, filtered with suction, washed twice with 95% ethanol, and dried to obtain 4.0 g of crude product. The crude product was recrystallized with chloroform to obtain 3.82 g of dark purple crystals, with a yield of 90.5%. 1 H-NMR (500MHz, CDCl 3, ppm) δ=8.631 (dd, 8H (Phen-H), J=...
Embodiment 2
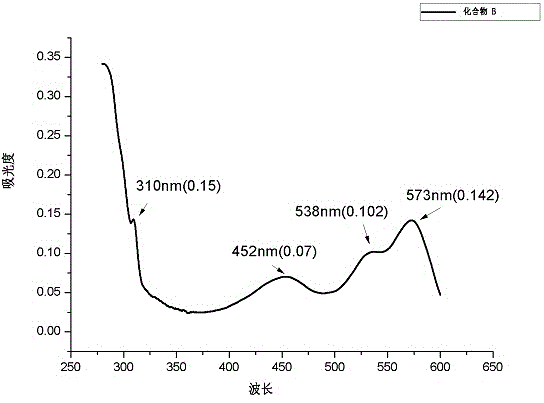
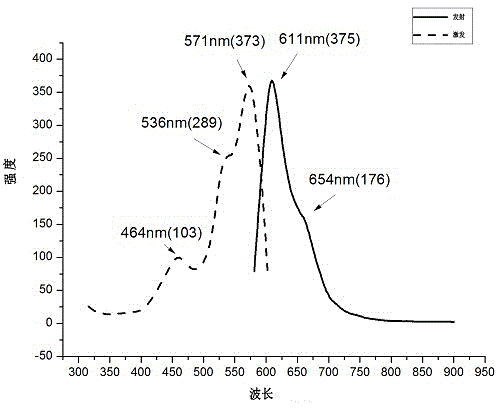
[0033] Example 2: Preparation of Fluorescent Compound B
[0034]
[0035] Fluorescent Compound B
[0036] According to Example 1, 3.75 g of 1,6,7,12-tetrachloro-N,N'-di-n-octyl-3,4,9,10-perylene diimide was used instead of 1,6,7, 12-Tetrachloro-N,N'-di-n-butyl-3,4,9,10-perylenediimide. Finally, 4.08 g of dark purple crystals were obtained, with a yield of 85.5%. 1 H-NMR (500MHz, CDCl 3 , ppm) δ=8.612 (dd, 8H (Phen-H), J=8.5Hz, 2.0Hz), 8.213 (s, 4H (Per-H)), 7.738 (t, 4H (Ar-H), J= 7.5Hz), 7.360~7.342 (m, 12H (Ar-H)), 7.043 (dd, 8H (Phen-H), J=7.0Hz, 2.0Hz), 4.091 (t, 4H, J=7.5Hz), 1.238 (m, 24H), 0.845 (t, 6H, J=7.0Hz).
[0037]
Embodiment 3
[0038] Example 3: Preparation of Fluorescent Compound C
[0039]
[0040] Fluorescent compound C
[0041] According to Example 1, 4.35 g of 1,6,7,12-tetrachloro-N,N'-dodecyl-3,4,9,10-perylenediimide was used instead of 1,6, 7,12-Tetrachloro-N,N'-di-n-butyl-3,4,9,10-perylenediimide. Finally, 4.73 g of deep purple crystals were obtained, with a yield of 89%. 1 H-NMR (500MHz, CDCl 3 , ppm) δ=8.591 (dd, 8H (Phen-H), J=8.5Hz, 2.0Hz), 8.221 (s, 4H (Per-H)), 7.713 (t, 4H (Ar-H), J= 7.5Hz), 7.386~7.335 (m, 12H (Ar-H)), 7.043 (dd, 8H (Phen-H), J=7.0Hz, 2.0Hz), 4.095 (t, 4H, J=7.5Hz), 1.450~1.238 (m, 40H), 0.876 (t, 6H, J=7.0Hz).
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com