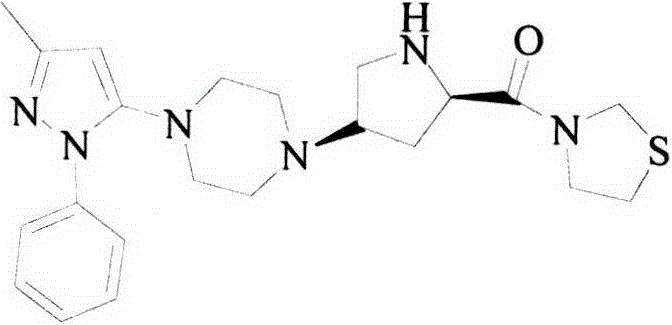
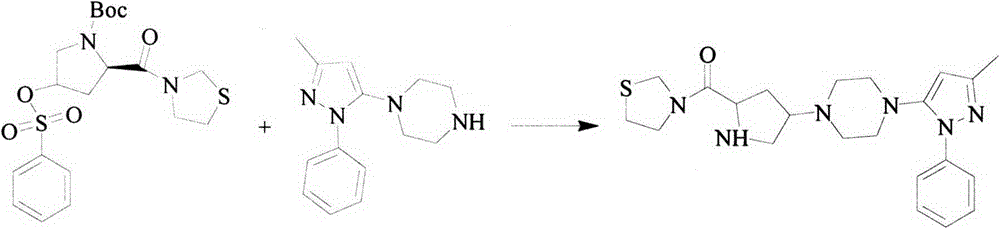
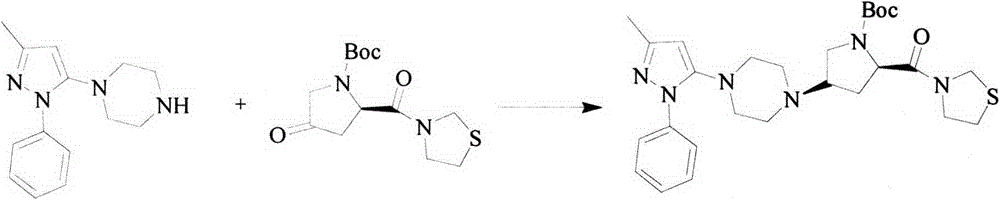
The preparation method of tiagliptin key intermediate 1-(3-methyl-1-phenyl-5-pyrazolyl)piperazine
A technology for ticagliptin and an intermediate, which is applied in the field of pharmaceutical preparation and can solve the problems of easily causing human body burns, low reaction yield, instability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 11-(3-methyl-1-phenyl-5--pyrazolyl)-4-tert-butoxycarbonylpiperazine
[0029] N-Boc-N acetoacetylpiperazine (200g, 0.7mol), phenylhydrazine (87.2g, 0.8mol), and 2400mL tetrahydrofuran were respectively added to a 3000mL four-neck flask, stirred and reacted at 30°C for 2h, and then 120mL of pyridine was added. After stirring for a while, Lawesson's reagent (329.2 g, 0.8 mol) was added and reacted overnight at 60° C., and the reaction was stopped after the completion of the reaction detected by TLC. Concentrate under reduced pressure, wash the oil in batches with ethyl acetate, combine several layers, wash with saturated sodium bicarbonate, dry and concentrate to give a yellow oil (228 g, 90%).
[0030] LC-MS(ESI)m / z343[M+H] + .
Embodiment 2
[0031] The preparation of embodiment 21-(3-methyl-1-phenyl-5--pyrazolyl)piperazine
[0032] The oil obtained above was fully dissolved with methanol until clear, and 400 mL of concentrated hydrochloric acid was added at 35° C., and the reaction was stopped after the completion of the reaction detected by TLC. Concentrate under reduced pressure, add 1000mL ethyl acetate to dissolve, wash with dilute hydrochloric acid, adjust the pH of the system until a large amount of solid precipitates, then wash the solid with DCM several times, dry and evaporate to dryness to obtain a gray solid (157.8g, 88%).
[0033] LC-MS(ESI)m / z243[M+H] + .
[0034] 1H NMR (500MHz, CDCl 3 ): δ2.26(3H, s), 2.8-3.4(8H, m), 5.67(1H, s), 7.21-7.24(1H, t), 7.27-7.42(2H, t), 7.74~7.77(2H , d).
Embodiment 3
[0035] Example 3 Preparation of 1-(3-methyl-1-phenyl-5--pyrazolyl)-4-tert-butoxycarbonylpiperazine
[0036] According to the operation of Example 1, the tetrahydrofuran solution was replaced with toluene solution, dried and concentrated to obtain a yellow oil with a yield of 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com