Three novel febuxostat medicament eutectic crystals and preparation method thereof
A febuxostat and drug technology is applied in the field of preparation of novel febuxostat organic drug co-crystals and drug co-crystals, and achieves the effects of good stability, high purity and simple and easy preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
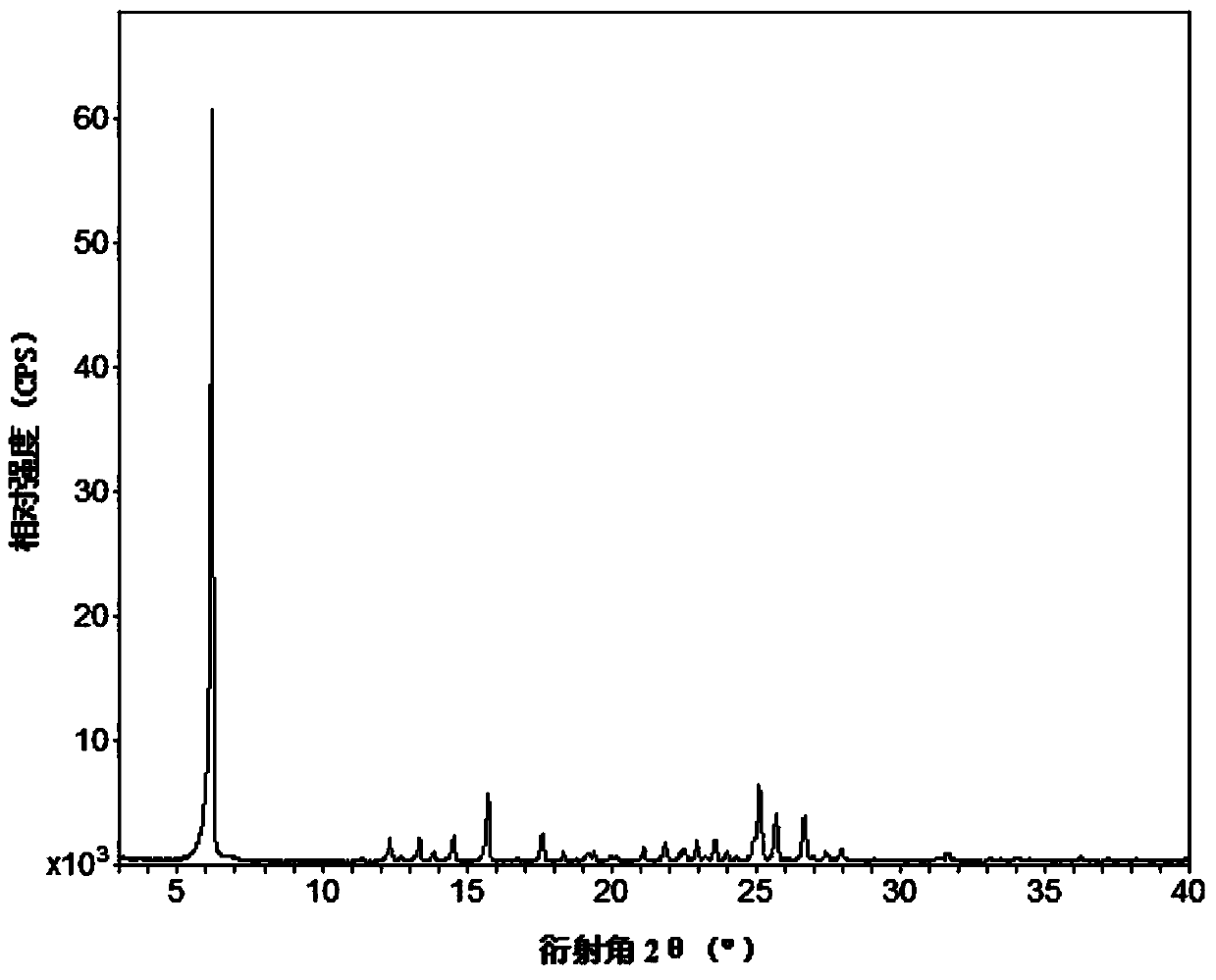
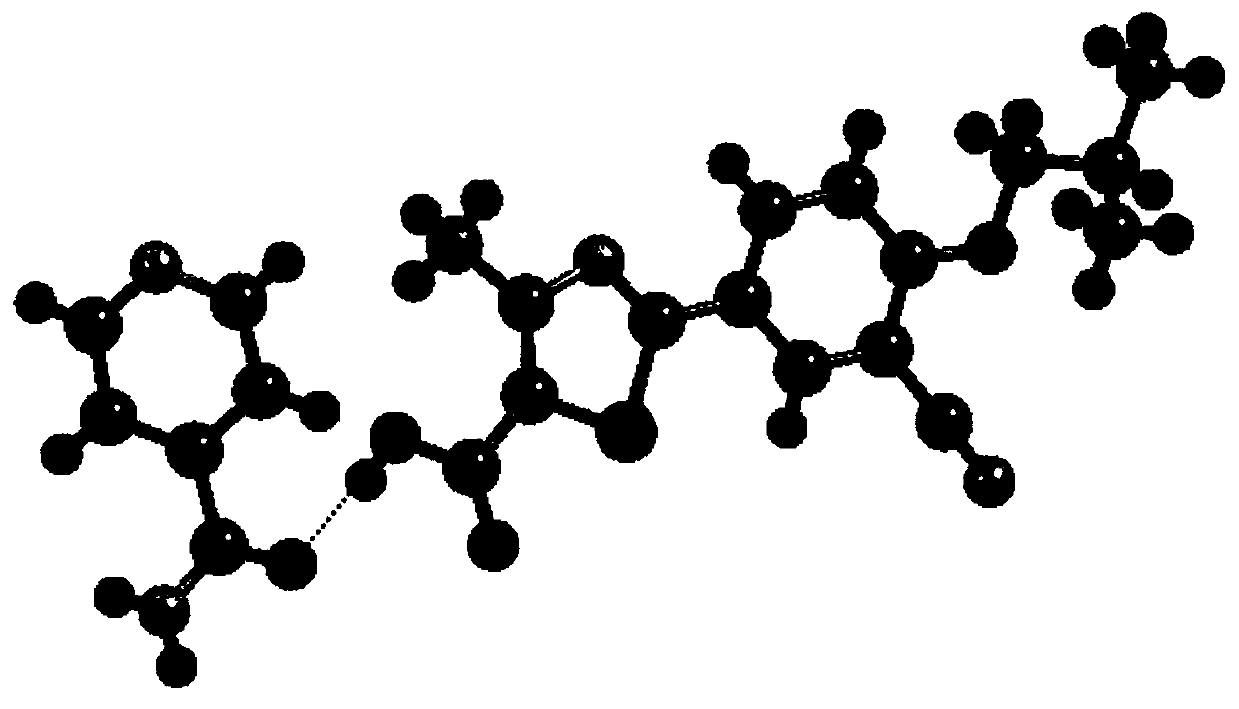
[0081] Example 1: Add 3.16g (10mmol) of febuxostat and 1.22g (10mmol) of isonicotine to 100mL of acetonitrile, stir, heat to reflux to 85°C, the solution is clear, continue to stir for 0.5h, take it out at room temperature After 4 hours of complete precipitation, filter with suction and dry to obtain a light yellow solid powder, which is febuxostat organic drug eutectic 1.
Embodiment 2
[0082] Example 2: Add 0.316g (1mmol) of febuxostat and 0.122g (1mmol) of isonicotine to 30mL of benzonitrile, stir, heat to reflux to 50°C, the solution is clear, continue to stir for 0.5h, then take it out at room temperature Stand still, and after 48 hours of complete precipitation, filter with suction and dry to obtain light yellow crystals, which are febuxostat organic drug eutectic 1.
Embodiment 3
[0083] Example 3: Add 0.316g (1mmol) of febuxostat and 0.122g (1mmol) of isonicotine to 40mL of benzyl nitrile, stir, heat to reflux to 70°C, the solution is clear, continue to stir for 0.5h, then take it out at room temperature Stand still, and after 48 hours of complete precipitation, filter with suction and dry to obtain light yellow crystals, which are febuxostat organic drug eutectic 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com