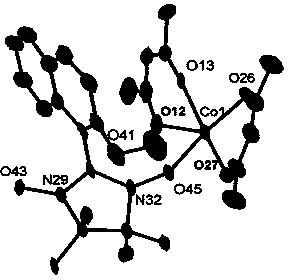
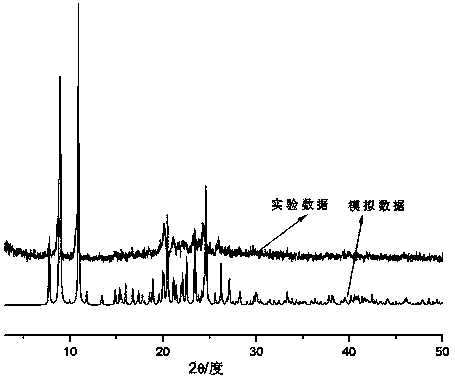
Nitroxyl free radical metal complex with naphthalene ring structure and preparation method of complex
A technology of nitroxide free radicals and metal complexes, applied in the direction of cobalt organic compounds, organic materials/organic magnetic materials, etc., can solve problems that have not been widely concerned by scientists, and achieve simple crystal growth conditions and synthesis temperature The effect of low cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0020] A kind of nitroxide radical metal complex of naphthalene ring structure, its chemical formula is: {Co(hfac) 2 (NIT-2-OMe-naph)} n , where hfac is hexafluoroacetylacetone, NIT-2-OMe-naph is 2-(2-methoxy-1-naphthyl)-4,4,5,5-tetramethylimidazoline-3-oxide- 1-oxygen free radical, its preparation method step is as follows:
[0021] 1) Synthesis of NIT-2-OMe-naph
[0022] Add 20 mL of anhydrous methanol to 4 mmol 2,3-dimethyl-2,3-dihydroxyaminobutane, stir the mixture for 20 minutes, then add 4 mmol 2-methoxynaphthaldehyde under stirring, room temperature Stirring and reacting for 1 day, a large amount of light yellow precipitates precipitated in the solution, and a light yellow powder was obtained after suction filtration; the powder was dissolved in 100 mL of chloroform and stirred in an ice bath for 10 minutes, then 1 g of sodium periodate was added and dissolved in Sodium periodate solution prepared in 50 mL of water was reacted at 0°C for 15 minutes, the purple organi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com