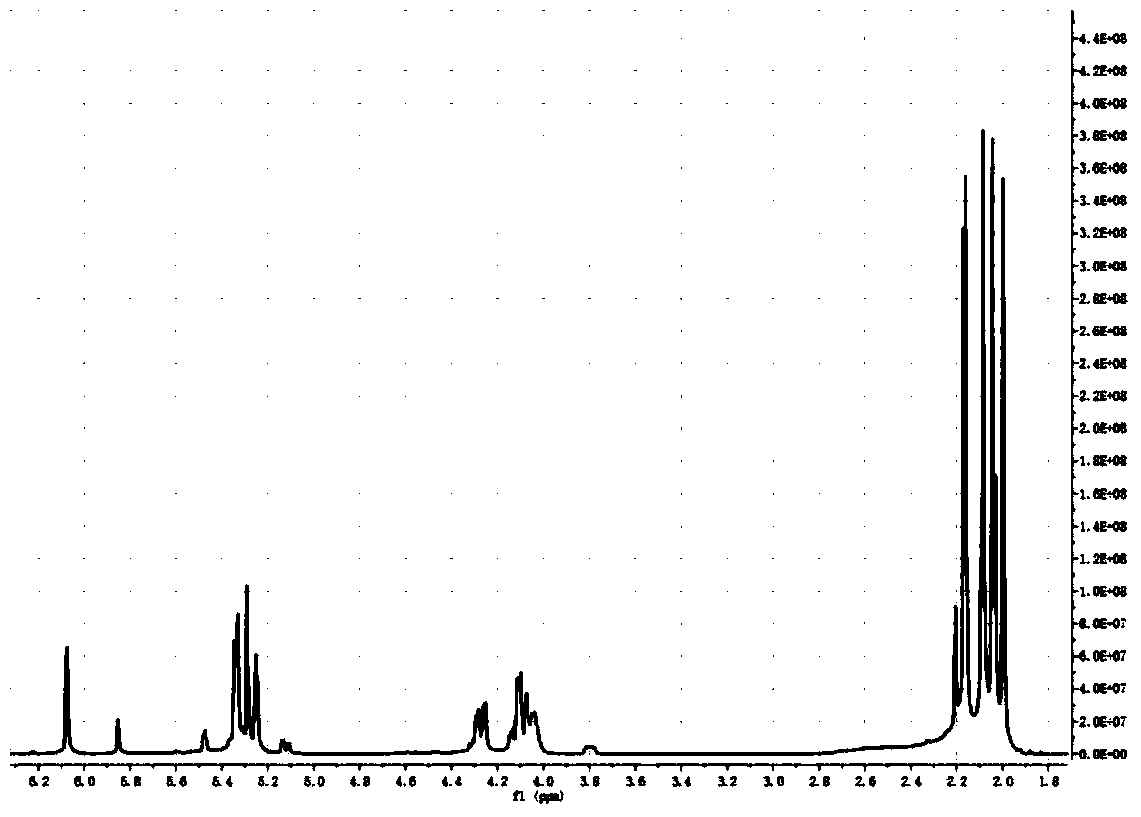
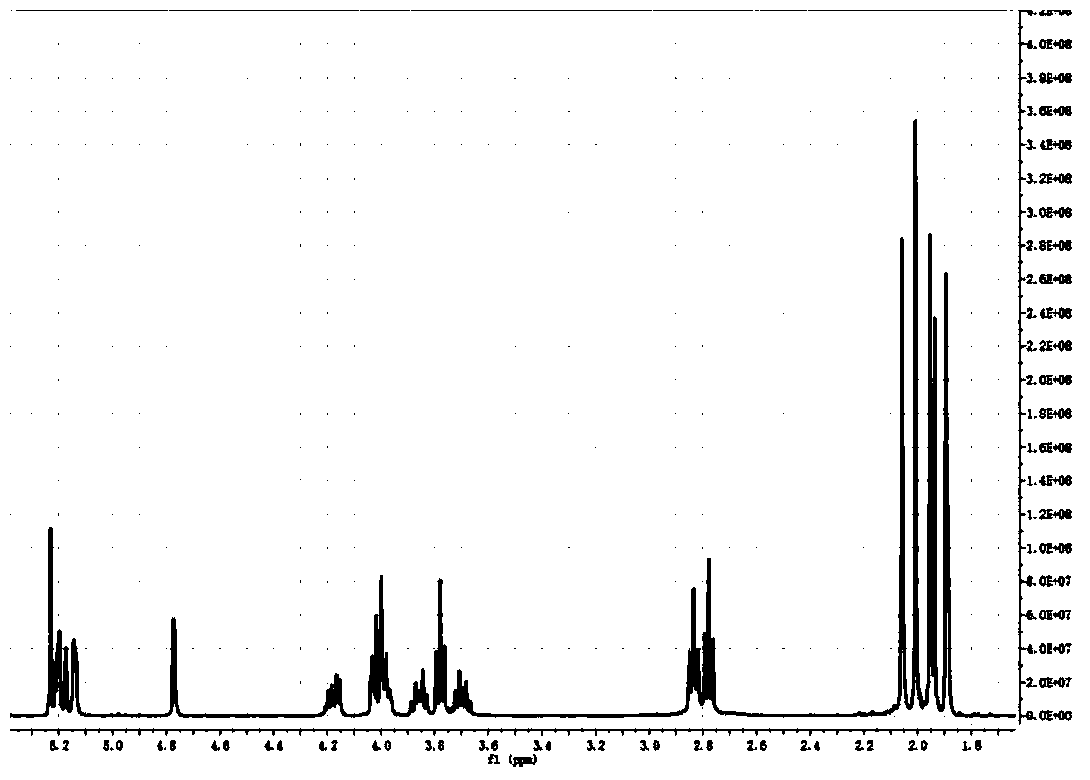
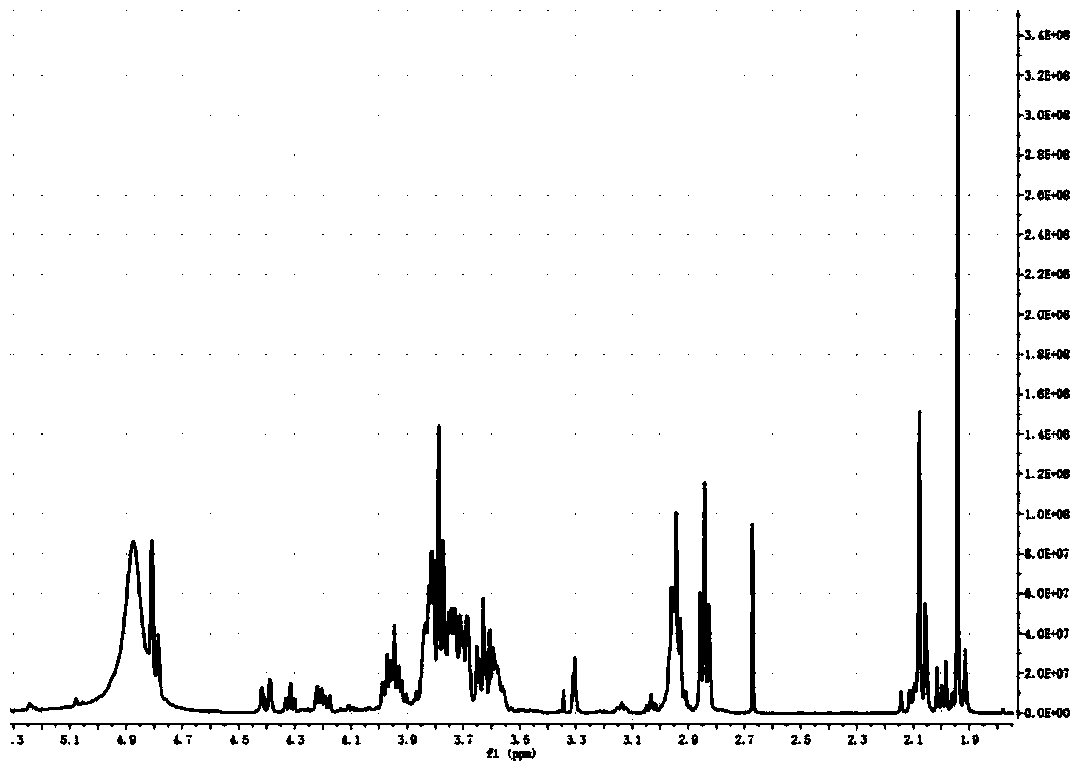
Synthesis method of nanoparticle surface modifier
A surface modifier and nanoparticle technology, applied in the field of nanomaterials, can solve problems such as lack of assembly types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1. At room temperature, 2.5g, at room temperature, 2.5g, 0.0139mol (1eq) powdered mannose (MW180.16) dissolved in 20mL NaSO 4 Add 10 mL, 0.083 mol (1.5 eq) of acetic anhydride to the dried pyridine, stir for 16 h and then 70°C rotary steam, extract with ethyl acetate, 2×100 mL saturated NaHCO 3 Solution, washed with 2×100mL saturated saline, then rotary evaporated at 40°C, and dried in vacuum; fully acetylated mannose was obtained.
[0022] 2.6.4mL BF 3 .Et 2 O (7.2g, 5eq, 51.2mmol) was added dropwise to 0°C, and the CH 2 Cl 2 solution (20mL, dry), and then the mixture can be reacted at room temperature for 24h (with constant stirring). with 30mL CH 2 Cl 2 Extraction, 20mL NaHCO 3 Washed twice, Na 2 SO 4 dry. The crude product can be separated with ethyl acetate:petroleum ether=1:1 to obtain a purified product.
[0023] 3. (0.2g, 0.37mmol) the product of step 2 was dissolved in 5mL methanol in a round bottom flask, added (0.03g, 0.55mmol) NaOMe stirred at roo...
Embodiment 2
[0027] 1. At room temperature, 2.5g, at room temperature, 2.5g, 0.0139mol (1eq) powdered mannose (MW180.16) dissolved in 20mL NaSO 4 Add 10 mL, 0.083 mol (1.5 eq) of acetic anhydride to the dried pyridine, stir for 16 h and then 70°C rotary steam, extract with ethyl acetate, 2×100 mL saturated NaHCO 3 The solution was washed with 2×100 mL saturated saline, then rotary evaporated at 40° C., and dried in vacuum to obtain fully acetylated mannose.
[0028] 2.6.4mL BF 3.Et 2 O (7.2g, 5eq, 51.2mmol) was added dropwise to 0°C, and the CH 2 Cl 2 solution (20mL, dry), and then the mixture can be reacted at room temperature for 24h (with constant stirring). with 30mL CH 2 Cl 2 Extraction, 20mL NaHCO 3 Washed twice, Na 2 SO 4 dry. The crude product can be separated with ethyl acetate:petroleum ether=1:1 to obtain a purified product.
[0029] 3. (0.2g, 0.37mmol) the product of step 2 was dissolved in 5mL methanol in a round bottom flask, added (0.03g, 0.55mmol) NaOMe stirred a...
Embodiment 3
[0033] 1. At room temperature, 2.5g, at room temperature, 2.5g, 0.0139mol (1eq) powdered mannose (MW180.16) dissolved in 20mL NaSO 4 Add 10 mL, 0.083 mol (1.5 eq) of acetic anhydride to the dried pyridine, stir for 16 h and then 70°C rotary steam, extract with ethyl acetate, 2×100 mL saturated NaHCO 3 Solution, washed with 2×100mL saturated saline, then rotary evaporated at 40°C, and dried in vacuum; fully acetylated mannose was obtained.
[0034] 2.6.4mL BF 3 .Et 2 O (7.2g, 5eq, 51.2mmol) was added dropwise to 0°C, and the CH 2 Cl 2 solution (20mL, dry), and then the mixture can be reacted at room temperature for 24h (with constant stirring). with 30mL CH 2 Cl 2 Extraction, 20mL NaHCO 3 Washed twice, Na 2 SO 4 dry. The crude product can be separated with ethyl acetate:petroleum ether=1:1 to obtain a purified product.
[0035] 3. (0.2g, 0.37mmol) the product of step 2 was dissolved in 5mL methanol in a round bottom flask, added (0.03g, 0.55mmol) NaOMe stirred at roo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com