PH-sensitive phospholipid medicinal material as well as preparation method and application thereof
A phospholipid, sensitive technology, applied in the application, pharmaceutical formulation, edible phospholipid composition, etc., can solve the problem that it is difficult to effectively improve the targeting of anticancer drugs, cannot change the accumulation of drug-loaded liposomes, and reduce anticancer drugs. Toxic and side effects, etc., to achieve the effect of good target aggregation ability, good charge reversal ability, and long cycle time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
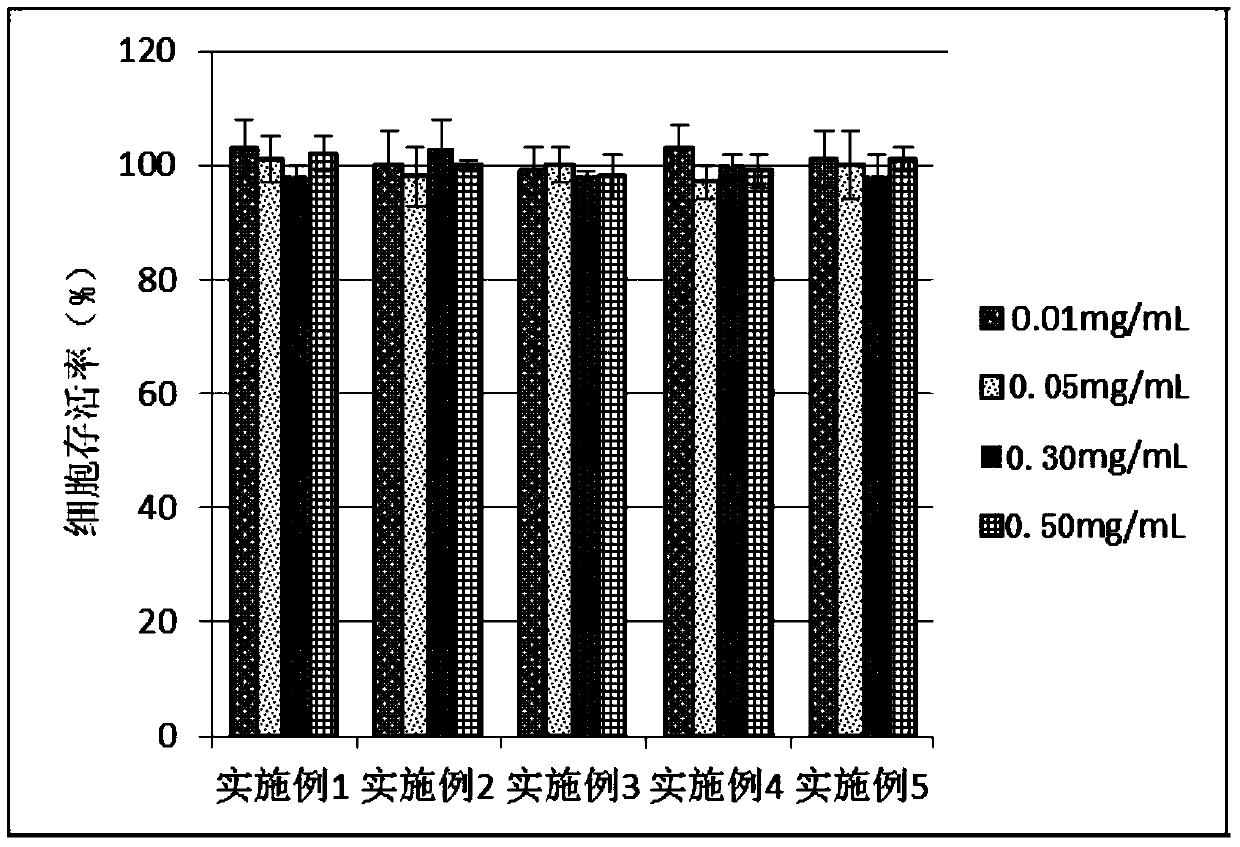
Examples
Embodiment 1
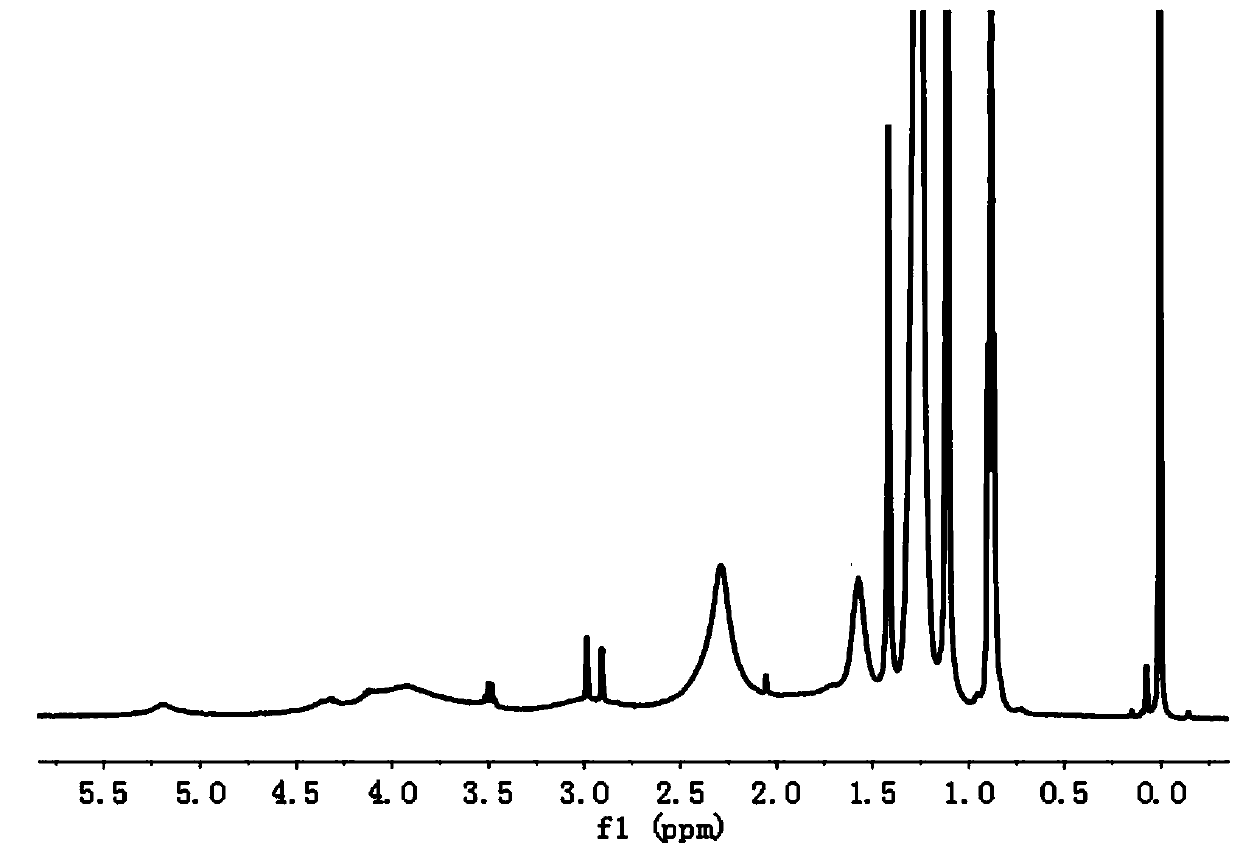
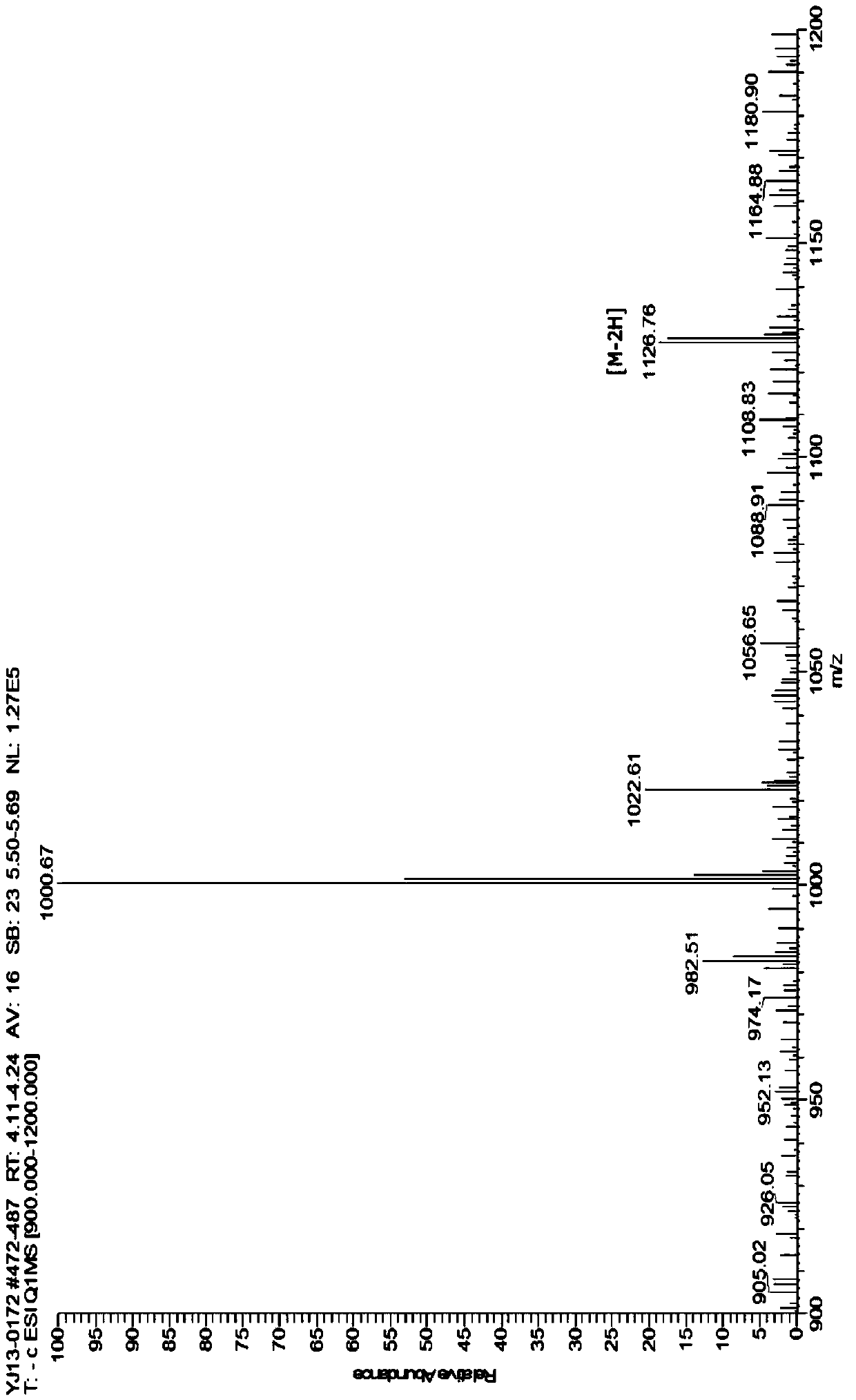
[0048] In this embodiment, the pH-sensitive phospholipid medicinal material is distearoylphosphatidylethanolamine-lysine-2,3-dimethylmaleic acid, and its structural formula is:
[0049]
[0050] Its preparation method is as follows:
[0051] (1) Amidation reaction of phosphatidylethanolamine and lysine with protective group
[0052] According to bis-tert-butoxycarbonyl-L-lysine (BOC-Lys-(BOC)-OH), condensing agent [equal molar 1-hydroxybenzotriazole (HOBT) and benzotriazole-N,N , N', N'-tetramethyluronium hexafluorophosphate (HBTU)], diisopropylethylamine (DIPEA) and distearoylphosphatidylethanolamine (DSPE) in molar ratios of 1.5:1, 3 :1, 6:1 metering of BOC-Lys-(BOC)-OH, HOBT, HBTU, DIPEA and DSPE;
[0053] Add BOC-Lys-(BOC)-OH, HOBT, HBTU, DIPEA and DSPE to chloroform to form a mixed solution. The amount of chloroform should make the concentration of DSPE in the mixed solution 1mmol / L. Stir the reaction at a speed of 52rpm for 24h, then filter the reaction solution an...
Embodiment 2
[0060] In this embodiment, the pH-sensitive phospholipid medicinal material is dioleoylphosphatidylethanolamine-lysine-2,3-diethylmaleic acid, and its structural formula is:
[0061]
[0062] Its preparation method is as follows:
[0063] (1) Amidation reaction of phosphatidylethanolamine and lysine with protective group
[0064] According to bisfluorenylmethoxycarbonyl-L-lysine (Fmoc-Lys-(Fmoc)-OH), cyclohexylcarbodiimide (DCC), 4-dimethylaminopyridine (DMAP) and dioleoylphosphatidylethanolamine The molar ratio of (DOPE) is 0.1:1, 1:1, 1:1 to measure Fmoc-Lys-(Fmoc)-OH, DCC, DMAP and DOPE respectively;
[0065] Add Fmoc-Lys-(Fmoc)-OH, DCC, DMAP and DOPE into dichloromethane to form a mixed solution. The amount of dichloromethane should make the concentration of DOPE in the mixed solution 10mmol / L. Under the protection of nitrogen, stir the reaction at a speed of 40rpm for 36h, then filter the reaction solution and concentrate the filtrate, add anhydrous ether to the conc...
Embodiment 3
[0072] In this embodiment, the pH-sensitive phospholipid medicinal material is 1-palmitoyl-2-oleoylphosphatidylethanolamine-lysine-2-methyl-3-ethylmaleic acid, and its structural formula is:
[0073]
[0074] Its preparation method is as follows:
[0075] (1) Amidation reaction of phosphatidylethanolamine and lysine with protective group
[0076] According to bisbenzyloxycarbonyl-L-lysine (Z-Lys(Z)-OH), diisopropylcarbodiimide (DIC), 4-dimethylaminopyridine (DMAP) and 1-palmitoyl-2 - Oleoylphosphatidylethanolamine (POPE) in molar ratios of 10:1, 10:1, 10:1 for metering Z-Lys(Z)-OH, DIC, DMAP and POPE;
[0077] Add Z-Lys(Z)-OH, DIC, DMAP and POPE to dichloromethane to form a mixed solution. The amount of dichloromethane should make the concentration of POPE in the mixed solution 50mmol / L. Stir the reaction at a speed of 60 rpm for 48 hours under protection, then filter the reaction solution and concentrate the filtrate, add acetone to the concentrated filtrate and place it...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com