Stable detection kit of total bile acid
A technology for detecting kits and total bile acids, applied in the field of medical immunology in vitro diagnosis, can solve the problems of difficult and long-term storage of NADH, poor reagent stability, short storage time, etc., and achieves improved accuracy, high sensitivity, and reduced dosage. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
[0036] The present examples 1-3 relate to a stable total bile acid detection kit, the kit includes reagent R1 and reagent R2, and the components and concentrations of the reagents R1 and R2 are shown in Table 1:
[0037] Table 1
[0038]
[0039] The TBA detection kits described in Examples 1 to 3 are applicable to various types of automatic biochemical analyzers. Taking Hitachi 7170 automatic biochemical analyzer as an example, its operation is shown in Table 2. Analysis method: Two-point endpoint method, that is, reagent R1; the amount of R2 is 270ul and 90ul respectively, and the sample volume is 4ul; 270ul reagent R1 is added to 4ul sample, and 90ulR2 is added after 5 minutes at 37°C, and the reading point is started after a delay of 60 seconds. The reading time is about 180 seconds; the detection wavelengths are respectively main wavelength 405nm and sub-wavelength 600nm.
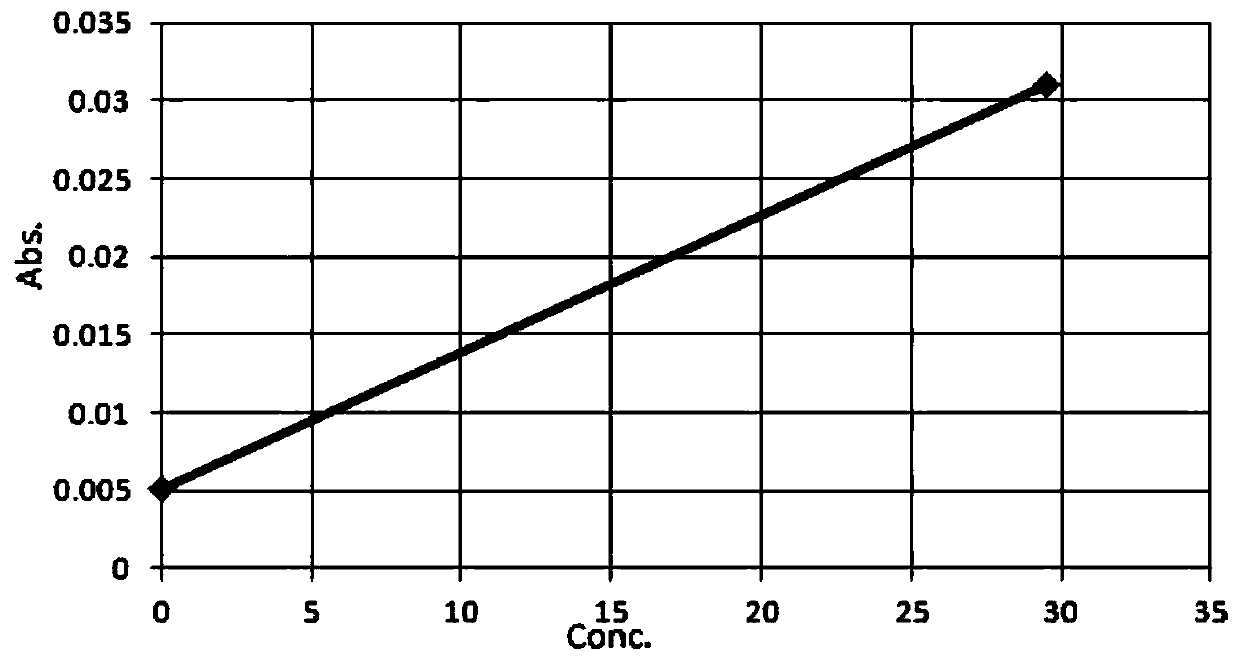
[0040] Adopt this reagent and above-mentioned assay method, adopt the curve of the TBA standard...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com