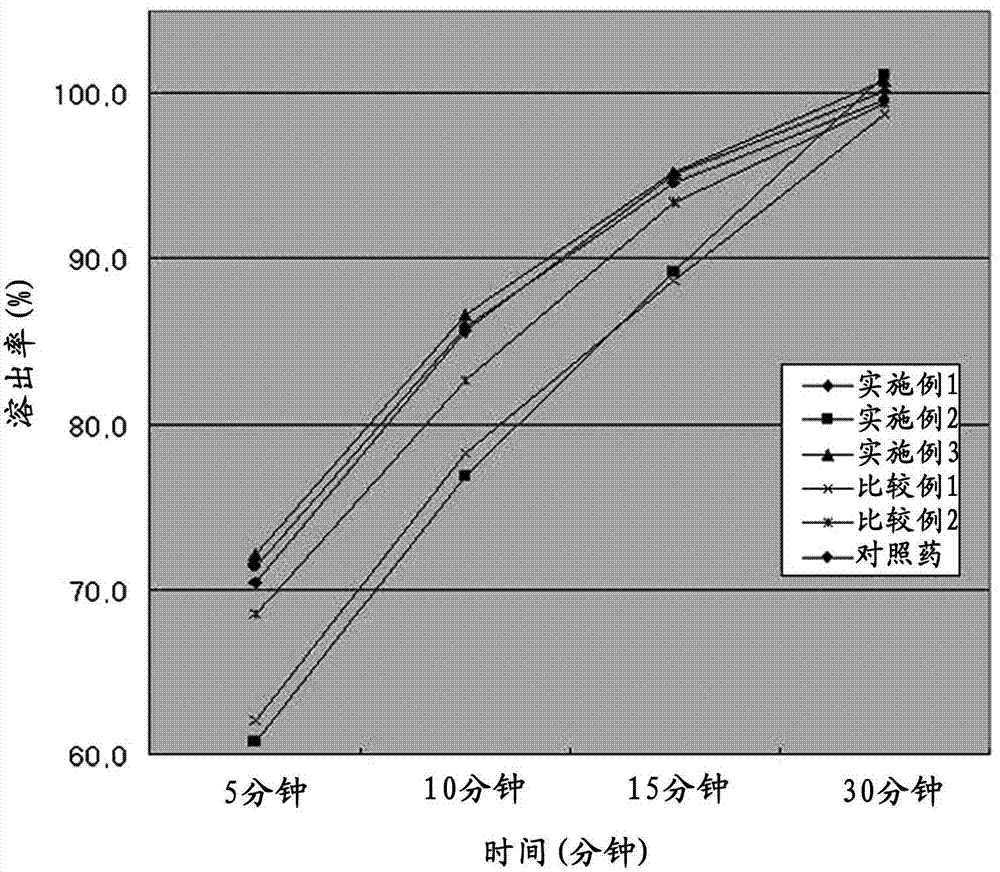
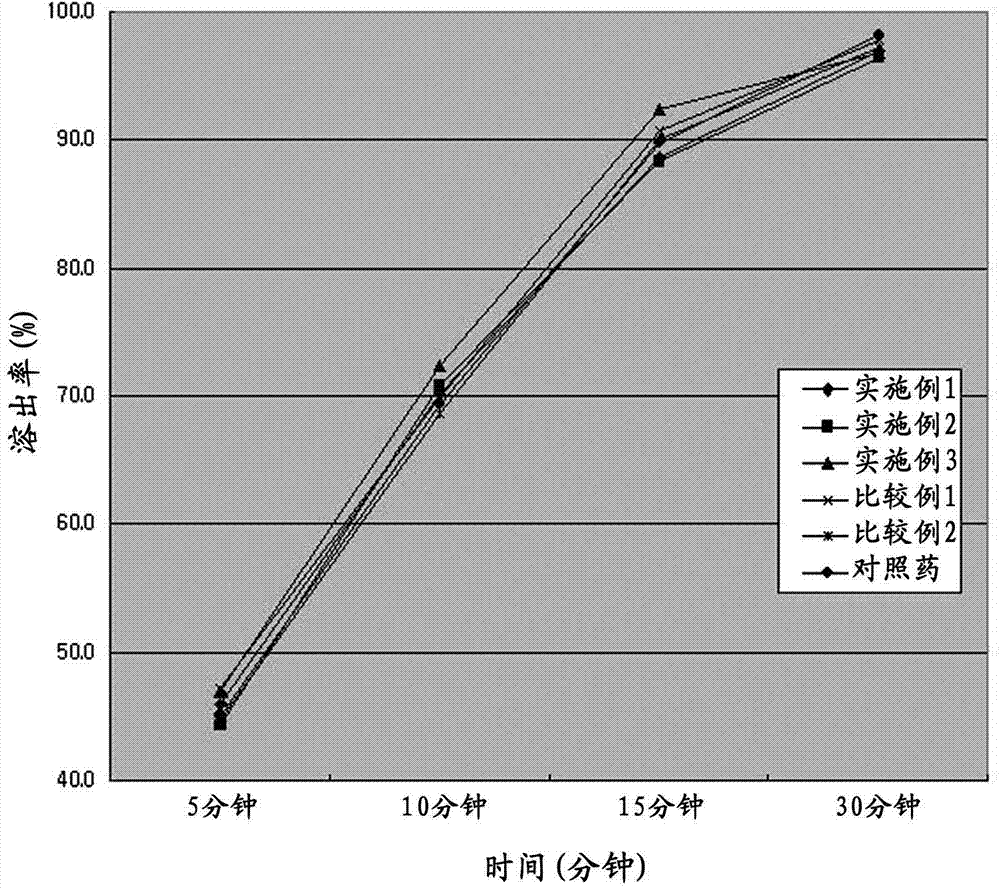
Bilayered tablet comprising repaglinide and meformin and preparation method thereof
A technology of metformin and double-layer tablets is applied in the directions of medical preparations containing active ingredients, pharmaceutical formulations, pills, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Preparation of Granules Containing Repaglinide
[0060] In order to prepare granules containing repaglinide, a combination solution in which povidone K30 and poloxamer 407 were dissolved in water was prepared with the composition shown in Table 1 below. Dissolve repaglinide, meglumine, and concentrated glycerin in the combination solution. Separately mix anhydrous calcium hydrogen phosphate, microcrystalline cellulose, pregelatinized starch, and red iron oxide, stir and dry it with a mixture dissolved in repaglinide, sieve with a mesh sieve to prepare wet granules, and put Add croscarmellose sodium and magnesium stearate and mix to prepare repaglinide granules.
[0061] Preparation of granules comprising metformin hydrochloride
[0062] With the composition shown in the following Table 1, metformin hydrochloride was stirred and dried with a combined solution of povidone K30 dissolved in ethanol and water, sieved with a mesh sieve to prepare wet granules, and stearic a...
Embodiment 2
[0067] Preparation of Granules Containing Repaglinide
[0068] In order to prepare granules containing repaglinide, a combination solution in which povidone K30 and poloxamer 407 were dissolved in water was prepared with the composition shown in Table 2 below. Dissolve repaglinide and meglumine in the combination solution. Separately mix anhydrous calcium hydrogen phosphate, microcrystalline cellulose, pregelatinized starch, and red iron oxide, stir and dry it with a mixture dissolved in repaglinide, sieve with a mesh sieve to prepare wet granules, and put Add croscarmellose sodium and magnesium stearate and mix to prepare repaglinide granules.
[0069] Preparation of granules comprising metformin hydrochloride
[0070] With the composition shown in Table 2 below, metformin hydrochloride was stirred and dried with a combination solution of povidone K30 and concentrated glycerin dissolved in ethanol and water, and was sieved with a mesh sieve to prepare wet granules. magnesi...
Embodiment 3
[0075] Preparation of Granules Containing Repaglinide
[0076] In order to prepare granules containing repaglinide, a combination solution in which povidone K30 and poloxamer 407 were dissolved in water was prepared with the composition shown in Table 3 below. Dissolve repaglinide, meglumine, and concentrated glycerin in the combination solution. Separately mix anhydrous calcium hydrogen phosphate, microcrystalline cellulose, pregelatinized starch, and red iron oxide, stir and dry it with a mixture dissolved in repaglinide, sieve with a mesh sieve to prepare wet granules, and put Add croscarmellose sodium and magnesium stearate and mix to prepare repaglinide granules.
[0077] Preparation of granules comprising metformin hydrochloride
[0078] With the composition shown in the following Table 3, metformin hydrochloride was stirred and dried with a combined solution of povidone K30 and concentrated glycerin dissolved in ethanol and water, and was sieved with a mesh sieve to p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com