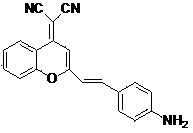
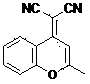
Application of fluorescent compound in A beta plaque imaging
A fluorescent compound and compound technology, applied in a method, in the field of fluorescent compounds, can solve the problems of little progress in Aβ fluorescent molecular probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: Synthesis of BPAD compounds.
[0050]
[0051] .
[0052] Synthetic intermediate 1
[0053] After 9.93 g (72.9 mmol) of o-hydroxyacetophenone was dissolved in 150 mL of ethyl acetate, 6.01 g (261.0 mmol) of sodium was added and reacted at room temperature for 3 hours. The solution changed from yellow to khaki. After the reaction, filter with suction, dissolve the resulting filter cake in 300 mL of water, adjust the pH to 7 with glacial acetic acid, and stir overnight. Suction filtration, obtain 4.97 g intermediate 1 . The yield was 32.6%.
[0054] .
[0055] Synthetic intermediate 2
[0056] intermediate 1 (4.44 g, 24.9 mmol) was dissolved in 50 mL of acetic acid, then 4 mL of concentrated sulfuric acid was added, and refluxed for half an hour. Pour the hot solution into 300 mL of ice water and adjust the pH to 7 with anhydrous sodium carbonate. After dichloromethane extraction, the organic phase was dried with anhydrous magne...
Embodiment 2
[0074] Embodiment 2: Determination of fluorescence excitation wavelength and fluorescence emission wavelength.
[0075] The compounds of the present invention have good fluorescence properties. In order to investigate the fluorescence properties of the compounds of the present invention, the specific implementation steps are: accurately weigh an appropriate amount of the compounds of the present invention, dissolve them in methanol, and dilute them to 1 μmol L with methanol -1 . Fluorescence detection was performed using a spectrofluorometer. Fix the excitation / emission wavelength and continuously scan the emission / excitation wavelength from 400-750 nm to draw the wave travel image. Table 1 shows the maximum excitation wavelength and maximum emission wavelength of some compounds in the examples of the present invention.
[0076] Table 1 Maximum fluorescence excitation wavelengths and maximum emission wavelengths of some compounds in the examples.
[0077] BPAD-1...
Embodiment 3
[0078] Embodiment three: Aβ 1-42 Fluorescence spectra of compounds after aggregate mixing.
[0079] The compounds of the present invention have the property of enhancing fluorescence after binding to Aβ aggregates. In order to investigate the change of the fluorescence spectrum behavior after the compound of the present invention is mixed with Aβ, the specific implementation steps are: accurately weigh an appropriate amount of the compound of the present invention, dissolve it in ethanol, and dilute it to 1 μmol L with PBS -1 . Fluorescence detection was performed using a spectrofluorometer. Fix the excitation / emission wavelength and continuously scan the emission / excitation wavelength from 400-750 nm to draw the wave travel image. Aβ 1-42 Proteins were incubated with Aβ aggregates in a 37°C water bath to simulate Aβ aggregates in the human brain. Compound (1 μmol L -1 ) and Aβ 1-42 aggregates (2.75 μmol L -1 ) were mixed, and the fluorescence detection was performed b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com