Nickel iron bimetallic methanation catalyst and preparation and application thereof
A methanation catalyst and catalyst technology, applied in catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc., can solve low-selectivity, pure nickel-based methanation catalyst Low activity and other problems, to achieve the effect of reducing the deactivation rate, reducing the metal loading, and saving costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Pseudoboehmite powder was added with 2% acetic acid solution, kneaded, extruded, dried in the air, and roasted in a muffle furnace at 500°C for 5 hours to obtain a carrier;
[0022] The nickel nitrate solution is impregnated on the carrier according to the proportion of 5wt% Ni, and left to stand for 10 minutes; the ferric nitrate solution prepared is impregnated on the above-mentioned nickel-containing carrier according to the proportion of 5wt% Fe, and left standing in the air for 24 hours, Until it is dry, it is dried in an oven at 80°C for 60 minutes; finally, it is calcined in a muffle furnace at 500°C for 5 hours to obtain catalyst No. 1.
Embodiment 2
[0039] Pseudo-boehmite powder was added with 2% acetic acid solution, kneaded, extruded, dried in the air, and calcined in a muffle furnace at 500°C for 5 hours to obtain an alumina carrier;
[0040] Immerse the magnesium nitrate solution according to the ratio on the carrier and let it stand for 10 minutes; dry it in an oven at 80-120°C for 60 minutes; bake it in a muffle furnace at 500°C for 2 hours to obtain a carrier containing 3wt% magnesium ;
[0041] The nickel nitrate solution is equally impregnated on the magnesium-containing carrier according to the proportion of 5wt% Ni, and left to stand for 10 minutes; the ferric nitrate solution prepared is excessively impregnated on the above-mentioned nickel-containing carrier according to the proportion of 5wt% Fe, and left to stand for 24 Hours, until dry, then dry in an oven at 80°C for 60 minutes; finally bake in a muffle furnace at 500°C for 5 hours to prepare catalyst No. 5.
Embodiment 3
[0043] Pseudo-boehmite powder was added with 2% acetic acid solution, kneaded, extruded, dried in the air, and calcined in a muffle furnace at 500°C for 5 hours to obtain an alumina carrier;
[0044] Immerse the lanthanum nitrate solution according to the proportion on the alumina carrier, and let it stand for 10 minutes; dry it in an oven at 80-120°C for 60 minutes; bake it in a muffle furnace at 500°C for 2 hours to obtain a lanthanum-containing 3wt% a;
[0045] The nickel nitrate solution is equally impregnated on the lanthanum-containing carrier according to the proportion of 5wt% Ni, and left to stand for 10 minutes; Let stand for 24 hours until dry, then dry in an oven at 80°C for 60 minutes; finally bake in a muffle furnace at 500°C for 5 hours to prepare catalyst No. 6.
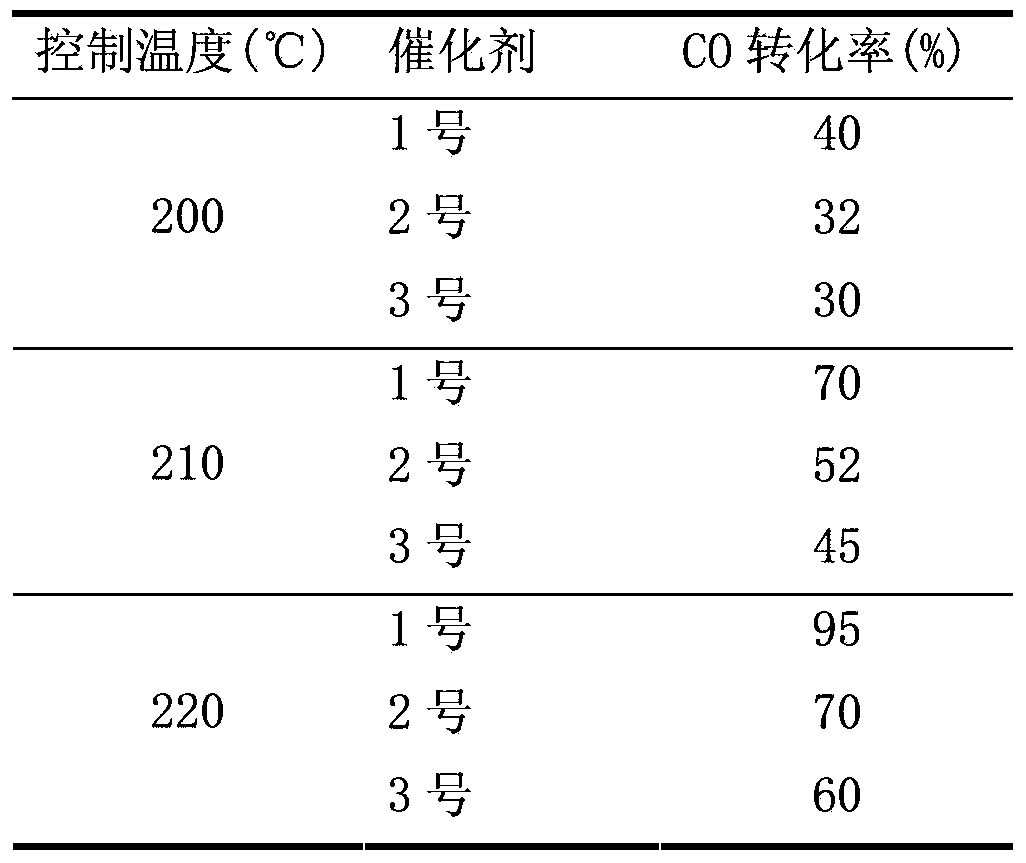
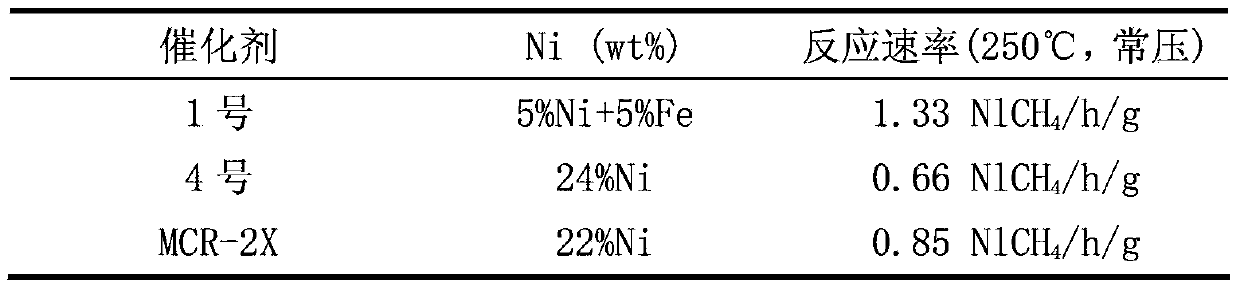
[0046] Take No. 1, No. 5 and No. 6 catalysts, crush them, sieve out 20-40 mesh particles, measure 1ml, about 0.65-0.7g, and put them into the reactor for methanation reaction evaluation. Before the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com