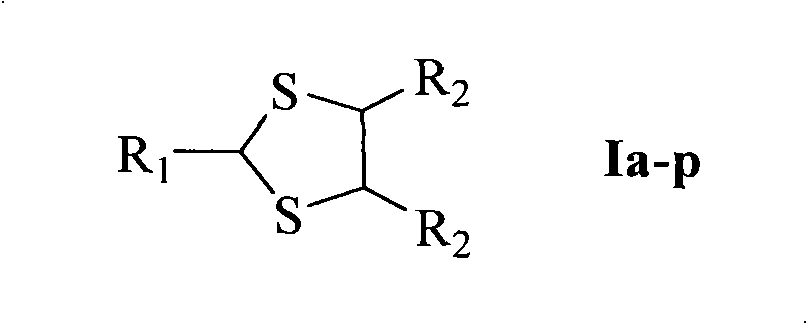
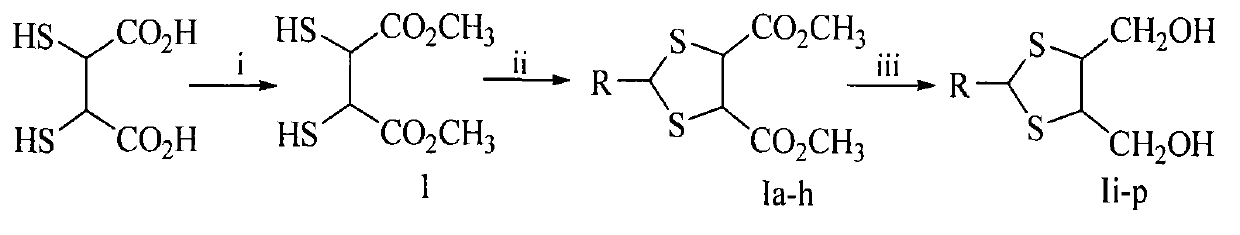
1,3-dithiolane derivatives, synthesis, nano structure, activity, and application thereof as lead dispelling agent
A compound, ia-p technology, applied in 1,3-dithiolane derivatives, its synthesis, nanostructure, activity and use as lead expelling agent, can solve the problem of many reaction steps and low yield , is not conducive to industrialization and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1 prepares dimethyl dimercaptosuccinate (1)
[0020] Dissolve 5.0 g (27.5 mmol) of dimercaptosuccinic acid (DMSA) powder in 200 mL of methanol, and feed freshly prepared dry HCl gas into the suspension under ice-cooling and stirring. After 2 hours of reaction, the reaction solution was clear, removed the ice bath, slowly returned to room temperature, and continued to stir for 6 hours. HCl and methanol were removed under reduced pressure, and the residue was washed with 10 mL of ether for three times. After filtration, the filter cake was recrystallized with 50% aqueous ethanol to obtain 4.5 g (77.6%) of the title compound as colorless needle crystals. ESI-MS(m / e): 211[M+H] + .
Embodiment 2
[0021] Example 2 Preparation of 4,5-dimethoxycarbonyl-2-(4-chlorophenyl)-1,3-dithiolane (Ia)
[0022] Dissolve 1.68g (12mmol) of 4-chlorobenzaldehyde and 2.11g (10mmol) of meso-dimethyl dimercaptosuccinate (1) in 50mL of methanol, and slowly add 1mL of concentrated sulfuric acid dropwise under stirring. After stirring at room temperature for 24 hours, with saturated NaHCO 3 The solution was adjusted to pH 7, concentrated under reduced pressure, 30 mL of ethyl acetate was added to dissolve the residue, washed three times with 50 mL of water, and then washed three times with 50 mL of saturated sodium chloride solution, the ethyl acetate phase was dried with anhydrous sodium sulfate for 2 hours, and filtered. The filtrate was concentrated under reduced pressure to obtain a pale yellow residue, which was purified by silica gel column chromatography (petroleum ether: ethyl acetate, 5:1) to obtain 2.25 g (68%) of the title compound as a colorless solid. ESI-MS(m / e): 333[M+H] + .Mp...
Embodiment 3
[0023] Example 3 Preparation of 4,5-dimethoxycarbonyl-2-(4-methylphenyl)-1,3-dithiolane (Ib)
[0024] Dissolve 1.44g (12mmol) of 4-methylbenzaldehyde and 2.11g (10mmol) of meso-dimethyl dimercaptosuccinate (1) in 50mL of dichloromethane, and slowly add 2mL of trifluoroacetic acid dropwise under stirring. After stirring at room temperature for 48 hours, with saturated NaHCO 3 The solution was adjusted to pH 7, concentrated under reduced pressure, 30 mL of ethyl acetate was added to dissolve the residue, washed three times with 50 mL of water, and then washed three times with 50 mL of saturated sodium chloride solution, the ethyl acetate phase was dried with anhydrous sodium sulfate for 2 hours, and filtered. The filtrate was concentrated under reduced pressure to obtain a colorless residue, which was purified by silica gel column chromatography (petroleum ether: ethyl acetate, 5:1) to obtain 2.05 g (66%) of the title compound as a colorless solid. ESI-MS(m / e): 313[M+H] + .Mp:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com