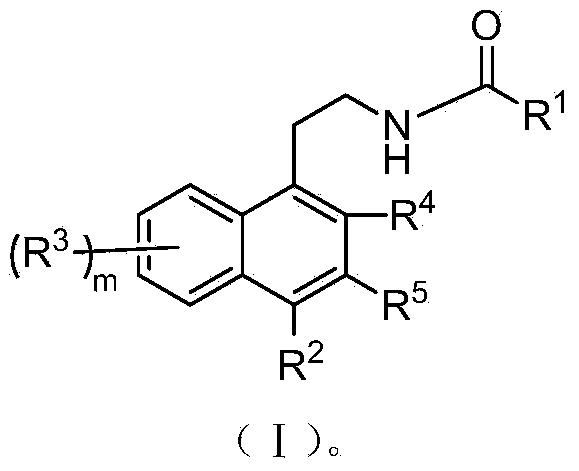
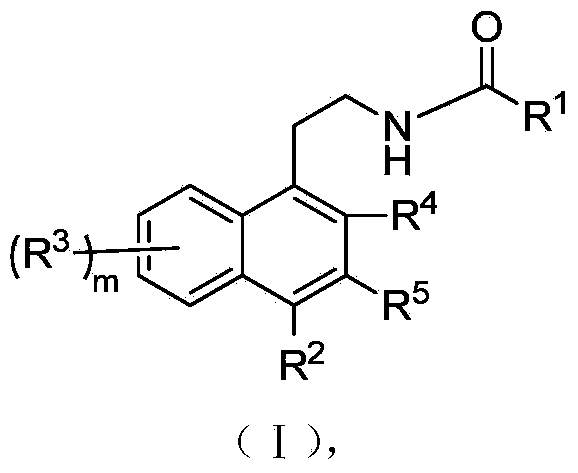
Naphthalene derivatives and application of naphthalene derivatives to medicine
A drug and pharmaceutical technology, applied in the application field of melatonin receptor agonist naphthalene compounds in the treatment of central nervous system dysfunction, can solve problems such as shortening sleep latency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0218] Example 1 N-(2-(4-methoxynaphthalen-1-yl)ethyl)acetamide
[0219]
[0220] Step 1) Synthesis of (E)-1-methoxy-4-(2-nitrovinyl)naphthalene
[0221] Combine 4-methoxy-1-naphthaldehyde (2.0g, 10.47mmol) and NH 4 OAc (828mg, 10.47mmol) was added to nitromethane (10mL), reacted for 12h at 120℃ in oil bath, stopped the reaction, diluted with ethyl acetate (60mL), washed with saturated brine (40mL×3), and separated The organic phase was then dried with anhydrous sodium sulfate. After filtration, the filtrate was spin-dried under reduced pressure, and purified by column chromatography (dichloromethane) to obtain the title compound as a red solid (2.20 g, 89.4%).
[0222] MS(ESI,pos.ion)m / z:230.1[M+1] + ;
[0223] 1 H NMR(CDCl 3 ,400MHz)δ8.78(d,J=13.6Hz,1H), 8.28(d,J=8.4Hz,1H), 8.26-8.20(m,2H), 8.18(d,J=8.0Hz,1H), 7.72-7.68 (m, 1H), 7.63-7.59 (m, 1H), 7.11 (d, J=8.0 Hz, 1H), 4.07 (s, 3H).
[0224] Step 2) Synthesis of 2-(4-methoxynaphthalene-1-yl)ethylamine
[0225] Add (E)-1-methoxy...
Embodiment 2
[0233] Example 2 N-(2-(4-fluoronaphthalene-1-yl)ethyl)acetamide
[0234]
[0235] Step 1) Synthesis of (E)-1-fluoro-4-(2-nitrovinyl)naphthalene
[0236] The title compound in this step was prepared by referring to the method described in step 1 of Example 1, namely 4-fluoro-1-naphthaldehyde (2.0g, 11.48mmol) and NH 4 OAc (885mg, 11.48mmol) was prepared by reaction in nitromethane (10mL). The crude product was subjected to silica gel column chromatography (dichloromethane), concentrated and dried to obtain the title compound as a grayish green solid (1.89g, 75.9%).
[0237] MS(ESI,pos.ion)m / z:218.0[M+1] + ;
[0238] 1 H NMR(400MHz,DMSO)δ8.76(d,J=13.2Hz,1H), 8.34(d,J=8.4Hz,1H), 8.21(d,J=13.2Hz,1H), 8.14-8.10(m , 2H), 7.79-7.71 (m, 2H), 7.45 (dd, J=10.4, 8.4 Hz, 1H).
[0239] Step 2) Synthesis of 2-(4-fluoronaphthalene-1-yl)ethylamine
[0240] The title compound of this step was prepared by referring to the method described in step 2 of Example 1, namely (E)-1-fluoro-4-(2-nitrovinyl)nap...
Embodiment 3
[0248] Example 3 N-(2-(4-(piperazin-1-yl)naphthalene-1-yl)ethyl)acetamide
[0249]
[0250] Step 1) Synthesis of 4-(piperazin-1-yl)-1-naphthaldehyde
[0251] 4-Fluoro-1-naphthaldehyde (10g, 57.41mmol), piperazine (14.84g, 172.24mmol) and potassium carbonate (15.85g, 114.82mmol) were added to DMF (50mL) in sequence, and the temperature was increased and refluxed for 12h . After cooling to room temperature, the solvent was removed by rotary evaporation under reduced pressure, 100 mL of dichloromethane was added, and washed with water (100 mL×3). After liquid separation, the organic phase was dried with anhydrous sodium sulfate. After filtration, the filtrate was spin-dried under reduced pressure and purified by column chromatography (DCM / MeOH(v / v)=20 / 1) to obtain the title compound as a yellow solid (13g, 94.2%).
[0252] MS(ESI,pos.ion)m / z:241.2[M+H] + ;
[0253] 1 H NMR(600MHz, CDCl 3 )δ10.23(s,1H),9.33(d,J=8.4Hz,1H), 8.19(d,J=8.4Hz,1H), 7.90(d,J=7.6Hz,1H), 7.67-7.64( m, 1H), 7.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com