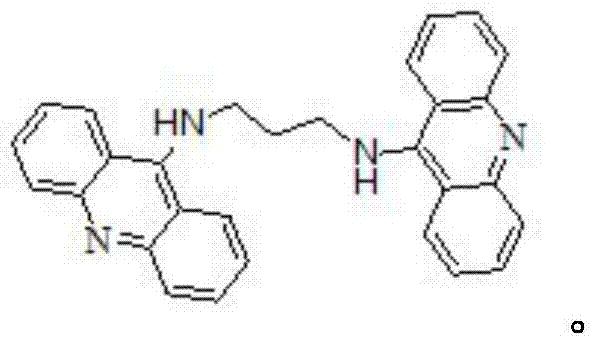
Bridge-linked acridine dimer and preparation method and application thereof
An acridine dimer and bridging technology, which is applied in the field of antineoplastic drugs and medicine, can solve the problems of high mortality, high recurrence rate, and high incidence rate, and achieve the effect of high response rate and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the preparation of bridging type acridine dimer
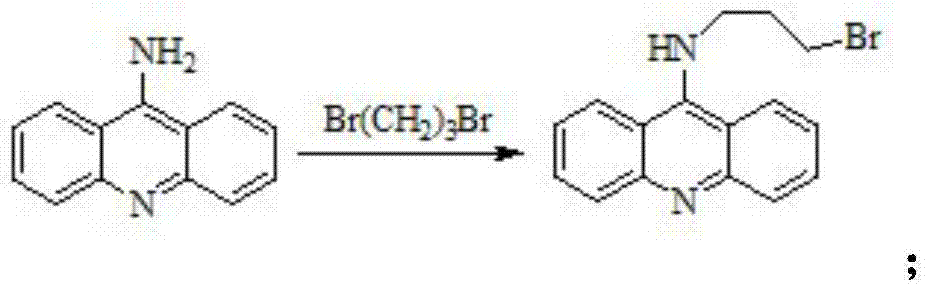
[0021] (1) Preparation of 9-bromopropylamine acridine:
[0022] Add 0.85g (4.4mmoL) 9-aminoacridine and 30mL acetone to a 100mL round bottom flask, stir until dissolved, then add 1mL (8.8mmoL) 1,3-dibromopropane, heat and stir in an oil bath to reflux, and react During the process, a yellow solid was gradually formed, and the reaction stopped after 24 hours. After the reaction mixture was cooled to room temperature, it was suction-filtered, and after recrystallization with acetone, yellow needle-like crystals were obtained, namely 9-bromopropylamine acridine, m.p.121-124°C, and the yield was over 70%.
[0023] Its reaction equation is as follows:
[0024]
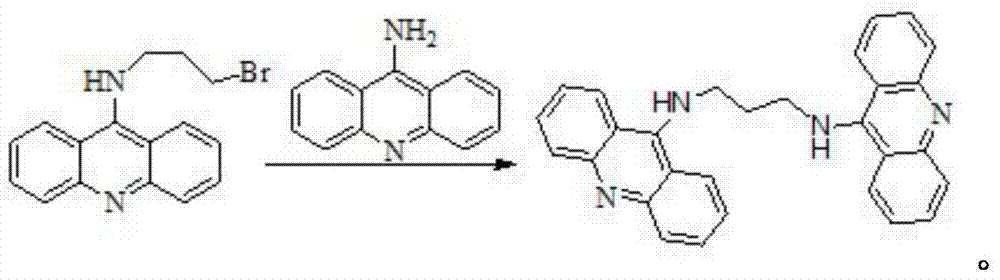
[0025] (2) Preparation of bridged acridine dimer:
[0026] Into a 50mL round bottom flask, add 0.35g (1mmoL) 9-bromopropylamine acridine and 20mL absolute ethanol in sequence, stir until dissolved, heat, stir and reflux in an oil bath for 10min, sl...
Embodiment 2
[0034] Example 2: In vitro tumor activity experiment
[0035] In vitro cytotoxicity assays were performed using the MTT method. The bridged acridine dimer obtained in Example 1 was reacted with the colon cancer Colo205 cell line for 24 hours, and the results are shown in Table 1.
[0036] Table 1 The half maximal effective concentration (IC) of bridged acridine dimer and colon cancer Colo205 cells 50 )
[0037]
[0038] From the results of Example 2, it can be seen that the bridged acridine dimer of the present invention has strong anti-tumor activity through in vitro anti-tumor experiments. The invention provides a new idea for researching and developing new antitumor drugs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com