Red thiazole heterocyclic disperse dye compound containing benzoate group and preparation and application thereof
A technology of disperse dyes and benzoic acid, applied in azo dyes, organic dyes, monoazo dyes, etc., can solve the problems of poor sublimation fastness and washing fastness, unsuitable for polyester fibers, and prone to color change, etc., to achieve Color fastness to washing, reduction of waste water discharge, full hue effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
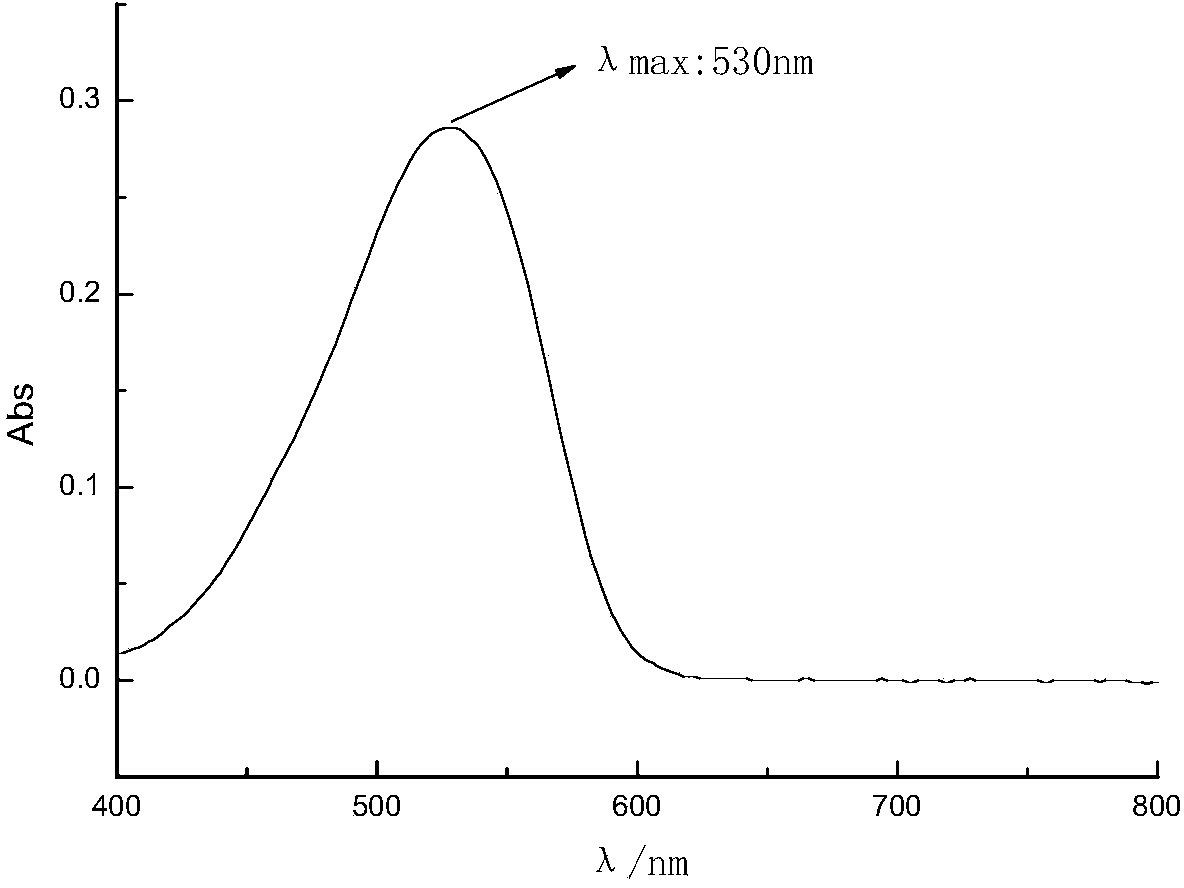
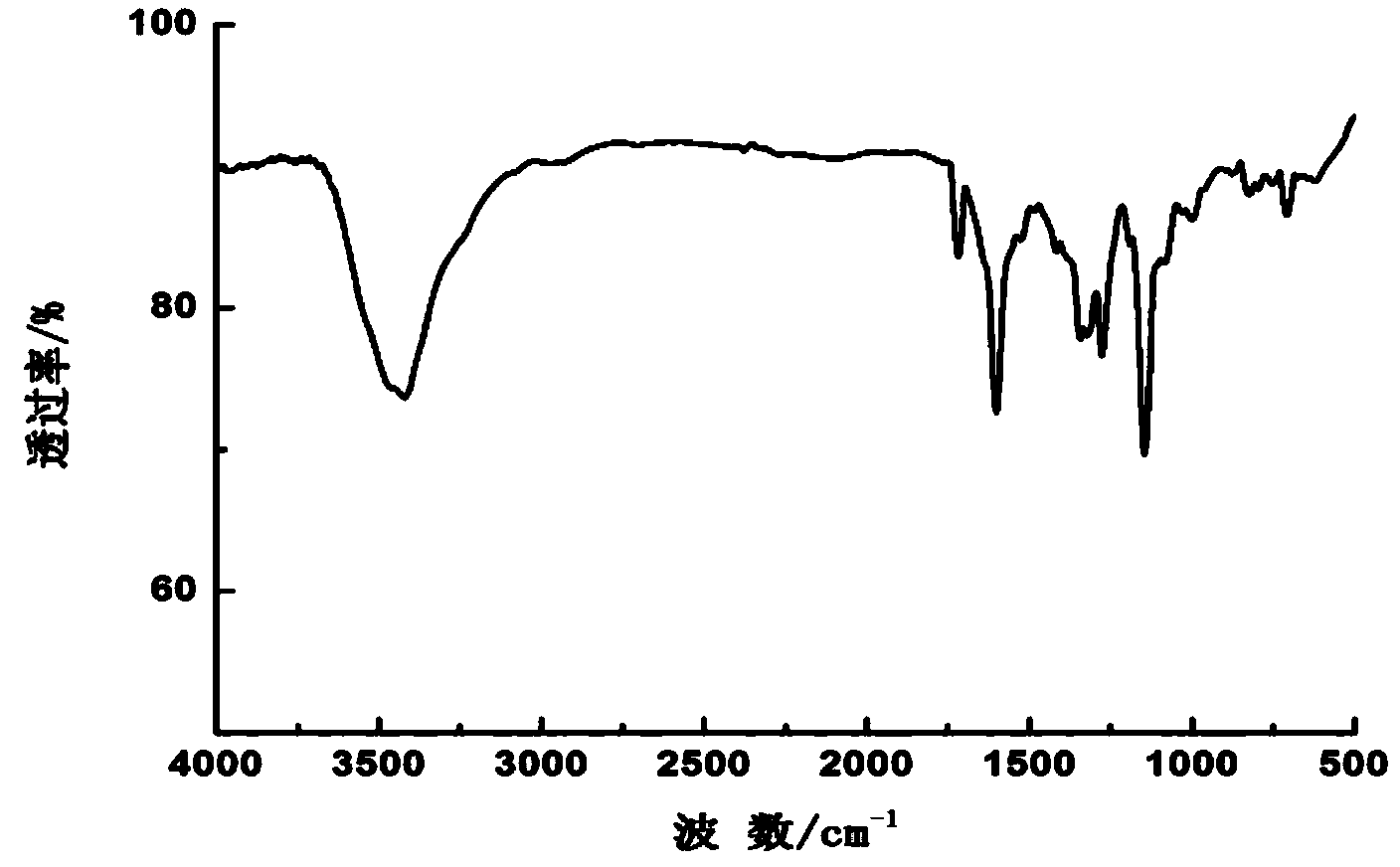
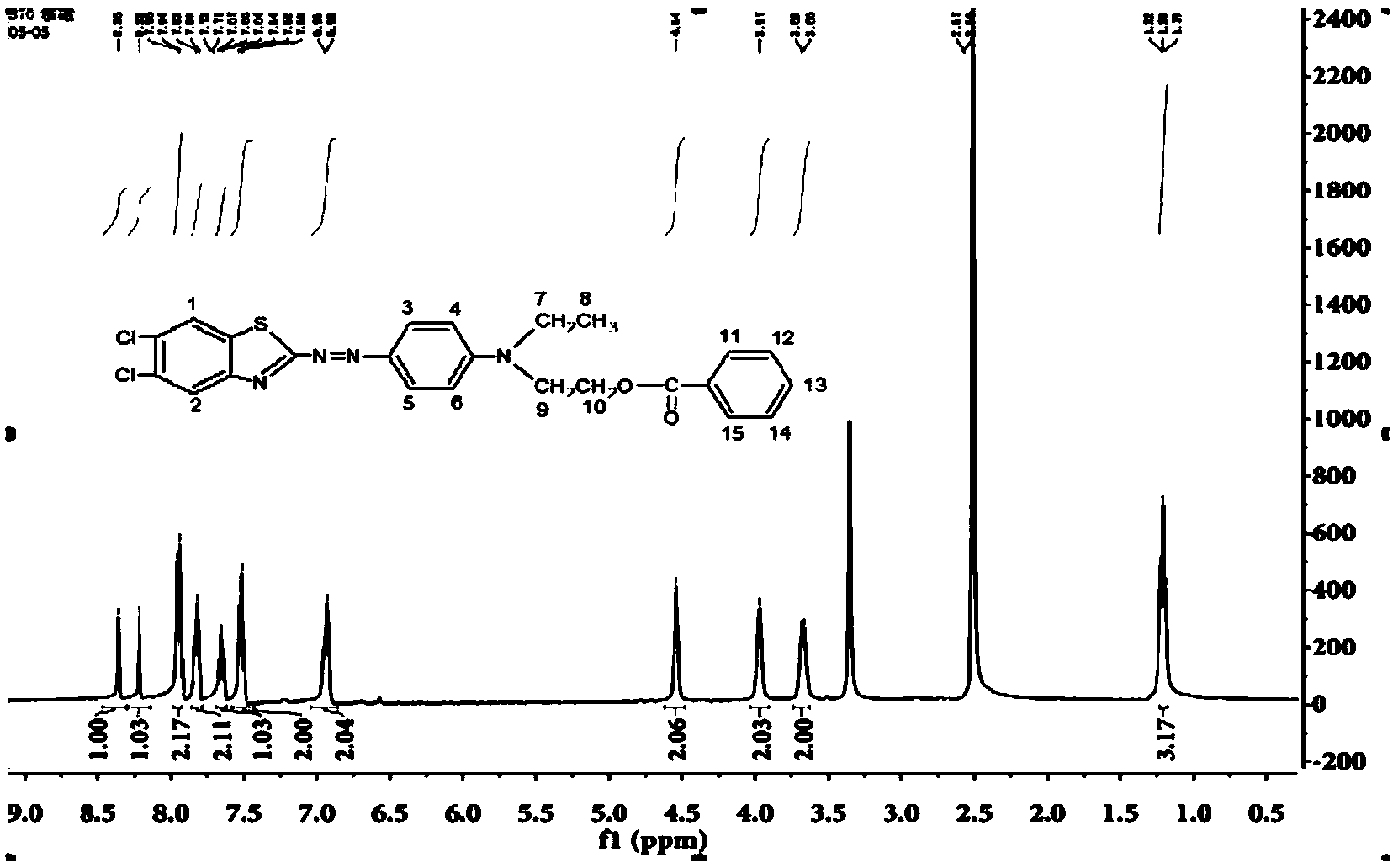
[0047] Synthesis and preparation of disperse dye compounds with the following molecular structure:
[0048]
[0049] (1) Preparation of diazonium salt:
[0050] Add 30g of 98% concentrated sulfuric acid into a 100ml three-necked flask, add 0.035mol of 5,6-dichloro-2-amino-benzothiazole under stirring, control the temperature at 10°C, then lower the temperature to below 10°C and add 0.035mol of Nitrosyl sulfuric acid. Take another three-necked flask, add 38g of water, slowly add 10g of 98% concentrated sulfuric acid, cool down to 0°C in an ice bath, then slowly add the mixture in the aforementioned three-necked flask dropwise, and keep the temperature at 0°C for 2 hours. Obtain the diazo component.
[0051] (2) Preparation of coupling components: In a three-neck flask equipped with a reflux condenser, water separator and stirrer, add 0.035mol benzoic acid, 0.035mol N-ethyl, N-hydroxyethylaniline and 100ml toluene in sequence , using 0.5g of p-toluenesulfonic acid as a catal...
Embodiment 2
[0058] Synthesis and preparation of disperse dye compounds with the following molecular structure:
[0059]
[0060] (1) Preparation of diazonium salt:
[0061] Add 30g of 98% concentrated sulfuric acid into a 100ml three-necked flask, slowly add 0.035mol of 5,6-dichloro-2-amino-benzothiazole under stirring, control the temperature at 20°C, then cool down to below 10°C and add 0.035mol of nitrosylsulfuric acid. Take another three-necked flask, add 38g of water, slowly add 10g of 98% concentrated sulfuric acid, cool down to 0°C in an ice bath, then slowly add the mixture in the aforementioned three-necked flask dropwise, and keep the temperature at 0°C for 2 hours. Obtain the diazo component.
[0062] (2) Preparation of coupling components: In a 100ml three-neck flask equipped with a reflux condenser, water separator and stirrer, add 0.035mol benzoyl chloride, 0.035mol N-ethyl, N-hydroxyethyl m-formazine in sequence Aniline and 100ml of toluene were heated under reflux fo...
Embodiment 3
[0070] Synthesis and preparation of disperse dye compounds with the following molecular structure:
[0071]
[0072] (1) Preparation of diazonium salt:
[0073] Add 30g of 98% concentrated sulfuric acid into a 100ml three-necked flask, slowly add 0.035mol of 5,6-dichloro-2-amino-benzothiazole under stirring, control the temperature at 20°C, then cool down to below 10°C and add 0.035mol of nitrosylsulfuric acid. Take another three-necked flask, add 38g of water, slowly add 10g of 98% concentrated sulfuric acid, cool down to 0°C in an ice bath, then slowly add the mixture in the aforementioned three-necked flask dropwise, and keep the temperature at 0°C for 2 hours. Obtain the diazo component.
[0074] (2) Preparation of coupling components: In a three-necked flask equipped with a reflux condenser, a water separator and an agitator, add 0.07mol benzoic acid, 0.035mol N-N-dihydroxyethylaniline and 100ml toluene in sequence, with 0.5g Use p-toluenesulfonic acid as a catalyst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com