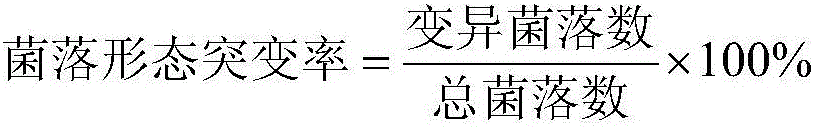The Attenuated Mutation Strain of Nocardia Amberatus and Its Application
A technology of Nocardia amberjack and Nocardia, which is applied in bacteria, antibacterial drugs, bacterial antigen components, etc., can solve the loss of feasibility of inactivated vaccine development routes and the development process of fish Nocardia vaccines. It is not ideal and cannot provide immune protection, so as to eliminate the possibility of spreading a large number of virulent pathogens, have a significant immune effect, and achieve a good control effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation of embodiment 1 attenuated live vaccine
[0022] (1) Mutation, screening and identification of the mutagenized attenuated strain of Nocardia japonica
[0023] 1. Preparation of Nocardia wild strain ZJ0503 bacterial suspension from yellowtail
[0024]In March 2005, our laboratory isolated a wild yellowtail Nocardia strain ZJ0503 (Huang Yucong et al. , Isolation and Identification of the Pathogen of Sarcoidosis in Pompano ovata, Journal of Guangdong Ocean University, 2008,28(4):49-53). The amberjack Nocardia ZJ0503 strain stored in a -80°C refrigerator was streaked on BHI solid medium, cultured upside down at a constant temperature of 28°C for 7 days, a single colony was picked and placed in a sterile phosphate buffer, and mixed to prepare bacterial suspension.
[0025] 2. UV mutagenesis
[0026] Pipette 0.1 ml of the ZJ0503 bacterial suspension and spread it on the modified solid medium plate of Nocardia yellowtail containing 0.3% to 0.5% lithium chlo...
Embodiment 2
[0053] Example 2 Detection of the pathogenicity of the mutagenized attenuated strain NSX1 of the yellowtail Nocardia
[0054] Under laboratory conditions, zebrafish was used as a model animal to detect the toxicity of the attenuated strain NSX1 of Nocardia amberii obtained through mutagenesis screening. The zebrafish (average body length 2.5-3cm, body weight about 0.2g) used in the experiment was first placed in a clean water tank to adapt to breeding for 1 week to eliminate abnormal individuals, and the mortality rate should be lower than 2%. Before the infection test, the test fish were divided into random groups, and each group was tested in parallel with 2 water tanks, and 50 fish were cultured in each tank (20 L). The test water tank uses tap water that has been oxygenated for more than 24 hours to replace 1 / 2 volume of aquaculture water every day, and the water temperature is 28±2°C. In the infection test, each group of test fish was treated with a certain gradient dose...
Embodiment 3
[0058] Example 3 Evaluation of the immune protection rate of Nocardiella mutagenic attenuated strain NSX1 from yellowtail
[0059] Zebrafish were used as experimental animals to carry out the immune protection test. The experimental zebrafish were randomly divided into 3 groups, each group had 3 parallel water tanks, 16 fish / box. The attenuated strain NSX1 was used as a live vaccine to immunize zebrafish by intraperitoneal injection, and the immunization dose was 10 7 CFU / tail. The control group was injected with sterile phosphate buffer. After 14 days of immunization, the zebrafish in each group were artificially infected and challenged with the wild strain ZJ0503 of Nocardia japonica, and the challenge dose was 10 6 CFU / tail. Observe and count the death numbers of the control group and the immune group within 14 days, and calculate the immune protection rate of each group (see Table 2).
[0060] Among them, the immune protection rate is calculated according to the follow...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com