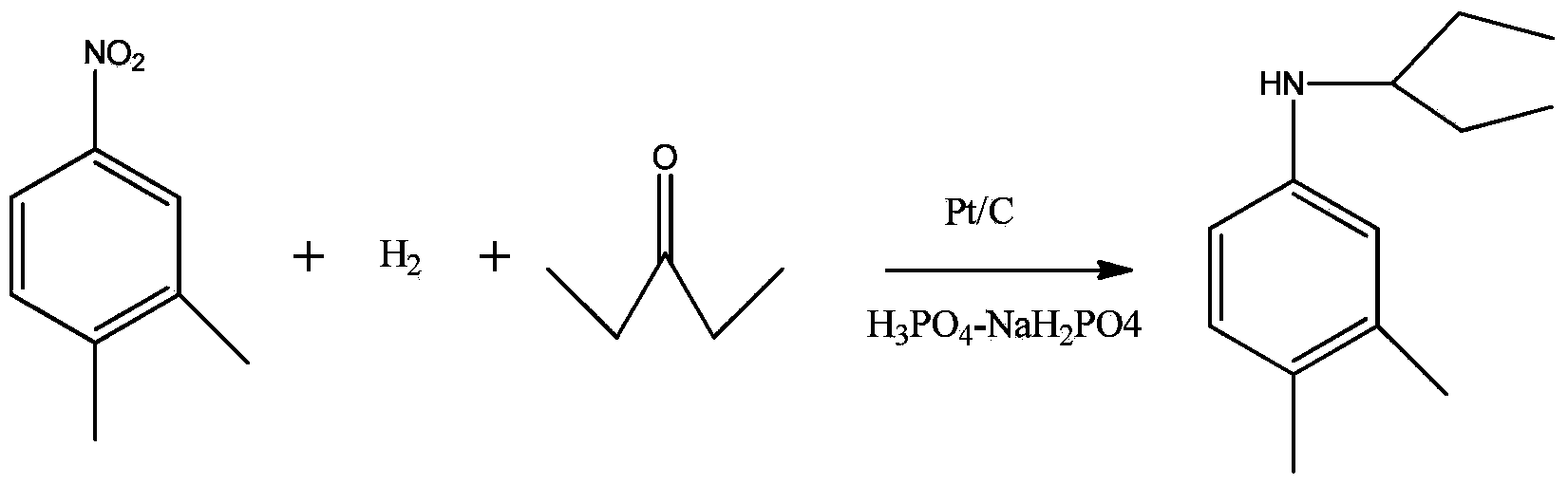Synthesis method of N-(1-ethyl propyl)-3, 4-dimethylaniline
A technology of dimethylaniline and ethylpropyl, which is applied in the synthesis field of N--3,4-dimethylaniline, can solve the problems of low total yield and long reaction route, and achieve stable product quality and reduced Difficulty, obvious effect of catalysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] 12.1 g (0.1 mol) of dimethylnitrobenzene, 13.95 g (0.15 mol) of 3-pentanone, 0.6 g of a platinum-carbon catalyst with a platinum mass loading of 4%, and phosphoric acid-sodium dihydrogen phosphate buffered at pH=3 Add 1ml of the solution into the high-pressure reactor together, replace the air with nitrogen three times, then replace the nitrogen with hydrogen three times, add hydrogen to 0.5MPa, raise the temperature to 50°C, and start stirring. After 25 minutes of reaction, the pressure of hydrogen no longer drops. Cool down, filter the kettle material, the filter cake is a platinum carbon catalyst, the filtrate is layered, the organic layer is distilled under reduced pressure, and the excess 3-pentanone is evaporated, and the raffinate is N-(1-ethylpropyl)-3,4 -Dimethylaniline, weighed, gas chromatographic analysis of the contents of each component, calculated yield. Implementation results: N-(1-ethylpropyl)-3,4-dimethylaniline content is 100%, raw material 3,4-dimeth...
Embodiment 2
[0015] Embodiment 2, phosphoric acid-sodium dihydrogen phosphate buffer solution pH changes
[0016] According to the same method as in Example 1, the pH of the phosphoric acid-sodium dihydrogen phosphate buffer solution is 2.5, and other experimental conditions and analysis methods are the same as in Implementation 1. Implementation results: N-(1-ethylpropyl)-3,4-dimethylaniline content is 99.5%, raw material 3,4-dimethylaniline content is 0, N-(1-ethylpropyl)- The yield of 3,4-dimethylaniline is 99.3%.
Embodiment 3
[0017] Embodiment 3, phosphoric acid-sodium dihydrogen phosphate buffer solution pH changes
[0018] According to the same method as in Example 1, the pH of the phosphoric acid-sodium dihydrogen phosphate buffer solution is 3.5, and other experimental conditions and analysis methods are the same as in Implementation 1. Implementation results: N-(1-ethylpropyl)-3,4-dimethylaniline content is 99.0%, raw material 3,4-dimethylaniline content is 0, N-(1-ethylpropyl)- The yield of 3,4-dimethylaniline is 98.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com