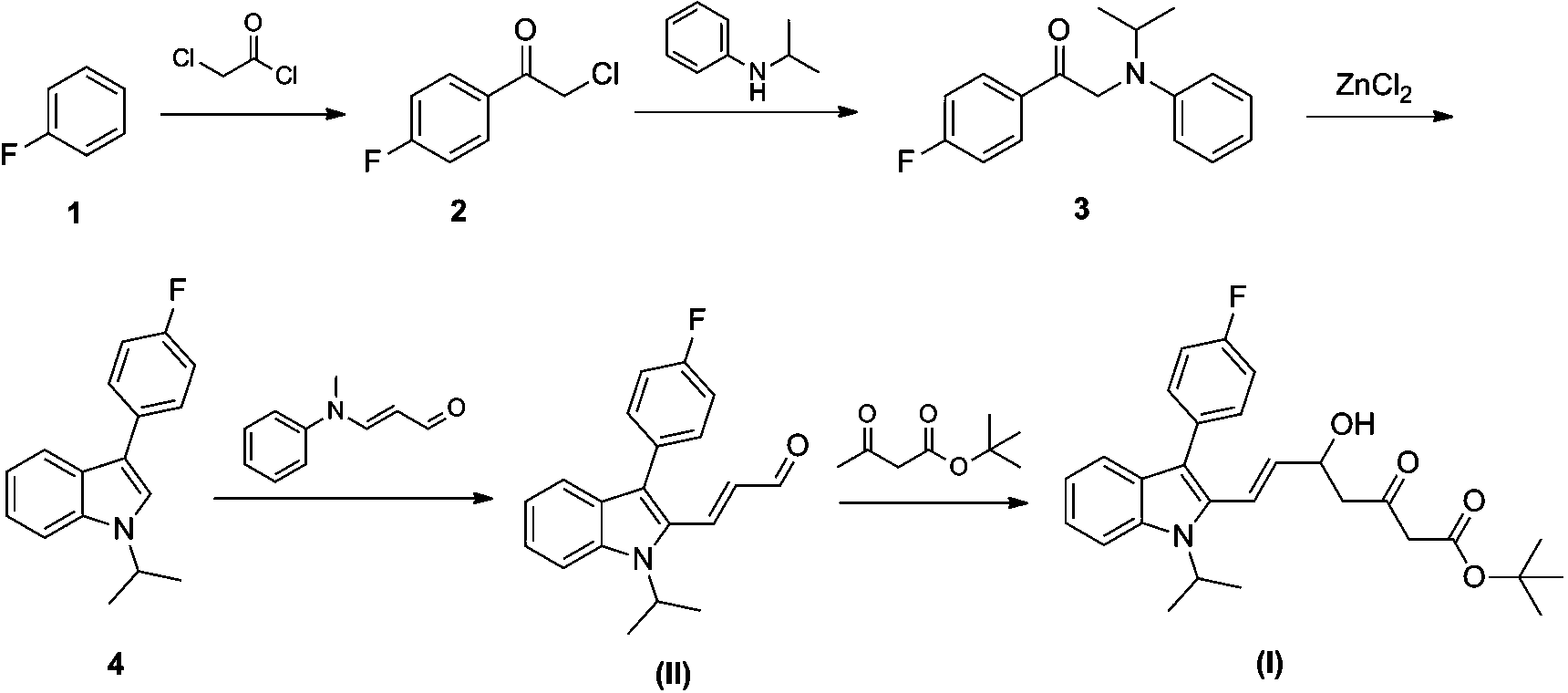
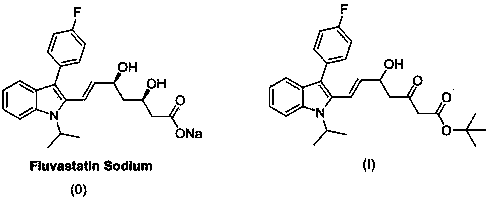
Improvement method of preparation technology of tert-butyl (E)-7-[3'-(4''-fluorophenyl)-1'-methylethyl-indol-2'-yl)-3-oxo-5-hydroxy-6-heptenoate
A preparation process, fluorophenyl technology, which is applied in the preparation of the cholesterol-lowering drug fluvastatin and the preparation of fluvastatin intermediate fluvasolone, which can solve the problems of high production cost, difficult recovery of solvent tetrahydrofuran, cumbersome operation, etc. , to achieve the effects of reducing production costs, improving recovery rates, and simplifying operating methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
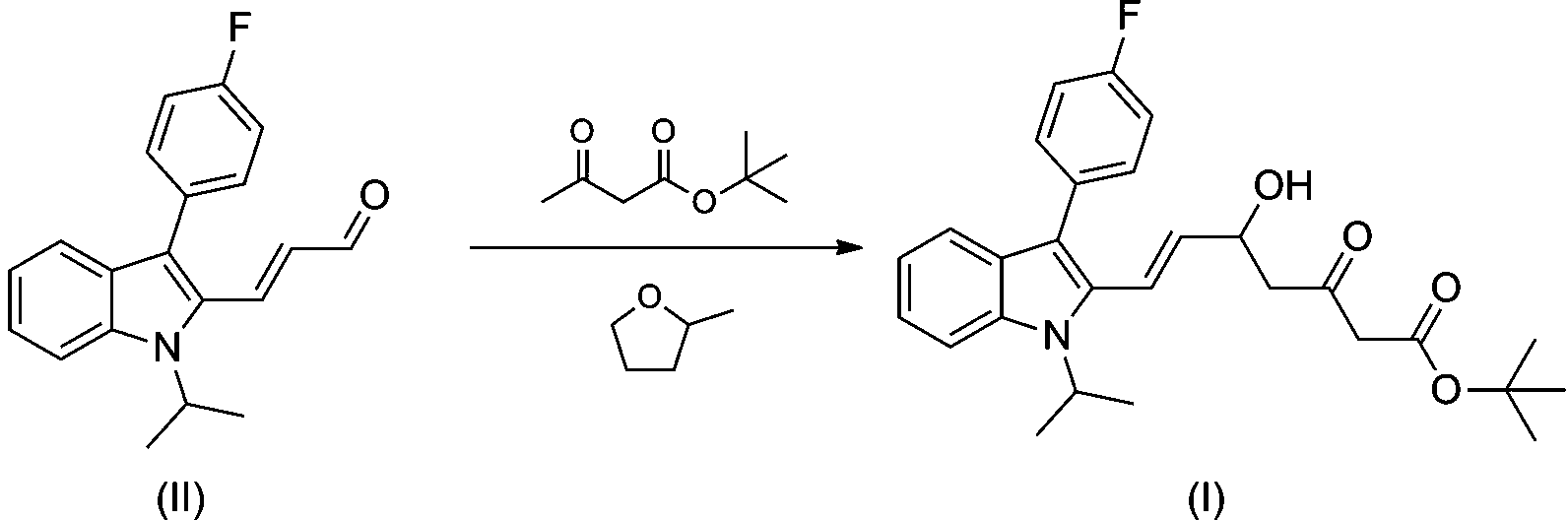
[0026] Add 450L of anhydrous 2-methyltetrahydrofuran into a dry nitrogen reactor, cool to 0°C, add 13.1 kg of 60% sodium hydride, and dropwise add 45.4 kg of tert-butyl acetoacetate, stir at 0°C for 30 minutes; then add dropwise 180 L of n-hexane solution of 1.6 mol / L n-butyllithium, continue stirring for 1 h; 84 kg (E)-3-[3'-(4''-fluorophenyl)-1'-isopropyl Indol-2'-yl]-2-propenal (II) in 100L of 2-methyltetrahydrofuran was slowly dropped into the reaction system, and the reaction was completed at -10°C for 2 hours.
[0027] The reaction solution was pumped into 250L hydrochloric acid of 1 mol / L, separated into layers, the organic phase was washed with saturated sodium chloride solution, dried over anhydrous magnesium sulfate, the solvent was evaporated to dryness under reduced pressure, and about 80% of 2-methyltetrahydrofuran was recovered to obtain The orange oil was dissolved in an appropriate amount of absolute ethanol, cooled and crystallized under stirring, and dried to...
Embodiment 2
[0032] Add 450L of anhydrous tetrahydrofuran into a reaction kettle with dry nitrogen, cool to 0°C, add 13.1 kg of 60% sodium hydride, and dropwise add 45.4 kg of tert-butyl acetoacetate, stir at -78°C for 30 minutes; then add dropwise 1.6 mol / L of n-butyl lithium in n-hexane solution 180 L, continue stirring for 1 h; 84 kg (E)-3-[3'-(4''-fluorophenyl)-1'-isopropylindole-2 The 100L tetrahydrofuran solution of '-yl]-2-propenal (II) was slowly dripped into the reaction system, and the reaction was completed at -10°C for 2 hours.
[0033] The reaction solution was pumped into 250L of 1 mol / L hydrochloric acid, most of the tetrahydrofuran was evaporated under reduced pressure, and about 50% was recovered, and the remaining reaction solution was extracted with 300L ethyl acetate, and the ethyl acetate layer was separated and washed with saturated chlorine Wash with sodium chloride solution, dry over anhydrous magnesium sulfate, evaporate the solvent to dryness under reduced pressu...
Embodiment 3
[0037] Add 105 L of anhydrous 2-methyltetrahydrofuran into a reaction kettle with dry nitrogen, cool to 0°C, add 3.4 kg of 60% sodium hydride, and dropwise add 11.4 kg of tert-butyl acetoacetate, and stir at -15°C for 30 minutes; Add 45 L of n-butyllithium n-hexane solution of 1.6 mol / L, and continue stirring for 1 h; A solution of indol-2'-yl]-2-propenal (II) in 25 L of 2-methyltetrahydrofuran was slowly dropped into the reaction system, and the reaction was completed at -10°C for 2 hours.
[0038] The reaction solution was pumped into 60L hydrochloric acid of 1 mol / L, separated into layers, the organic phase was washed with saturated sodium chloride solution, dried over anhydrous magnesium sulfate, the solvent was evaporated to dryness under reduced pressure, and about 80% of 2-methyltetrahydrofuran was recovered to obtain The orange oil was dissolved in an appropriate amount of absolute ethanol, cooled and crystallized under stirring, and dried to obtain 26.4 kg of a browni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com