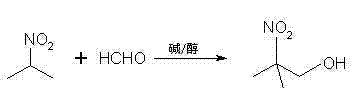
Method for preparing 2-nitro-2-methyl-1-propanol
A technology of nitropropane and methyl, which is applied in the field of preparation of 2-nitro-2-methyl-1-propanol, can solve the problems of complicated operation, low yield and high energy consumption, and achieve simple process and high product quality. High yield and reduced energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Dissolve 1g of potassium hydroxide in 25ml of methanol to form a solution, then add 30g (1mol) of paraformaldehyde into the solution, stir until dissolved, and form a solution for later use.
[0024] Then add 89g (1mol) 2-nitropropane into a 500ml flask, slowly drop the solution into the flask while stirring, and the dropping time is 30min. Then stirred and reacted at 35° C. for 1 h; after the reaction was completed, concentrated sulfuric acid was added dropwise to neutralize to a pH value of 4.5, and filtered.
[0025] The filtrate was distilled under reduced pressure to remove low-boiling impurities such as methanol and unreacted 2-nitropropane to obtain 113.4 g of 2-nitro-2-methyl-1-propanol crystals. The gas chromatogram of this crystal was consistent with that of 2- The standard spectrogram of nitro-2-methyl-1-propanol is consistent, and its yield is 95.3% after detection.
Embodiment 2
[0027] Dissolve 1g of triethylamine in 25ml of methanol to form a solution, then add 30g (1mol) of paraformaldehyde into the solution, stir until dissolved and form a solution for later use.
[0028] Then add 89g (1mol) 2-nitropropane into a 500ml flask, slowly drop the solution into the flask while stirring, and the dropping time is 30min. Then stirred and reacted at 45°C for 1.5h; after the reaction was completed, concentrated sulfuric acid was added dropwise to neutralize to a pH value of 3.5, and filtered.
[0029] The filtrate was distilled under reduced pressure to remove low-boiling impurities such as methanol and unreacted 2-nitropropane to obtain 111.7 g of 2-nitro-2-methyl-1-propanol crystals. The gas chromatogram of the crystals was consistent with that of 2- The standard spectrum of nitro-2-methyl-1-propanol is consistent, and its yield is 93.9% after detection.
Embodiment 3
[0031] First dissolve 1g of sodium hydroxide in 100ml of methanol to form a solution, then add 30g (1mol) of paraformaldehyde to the solution, stir until dissolved and form a solution for later use.
[0032] Then add 89g (1mol) 2-nitropropane into a 500ml flask, slowly drop the solution into the flask while stirring, and the dropping time is 1h. Then stirred and reacted at 20°C for 3h; after the reaction was completed, concentrated sulfuric acid was added dropwise to neutralize to a pH value of 4, and filtered.
[0033] The filtrate is subjected to vacuum distillation to remove low-boiling impurities such as methanol and unreacted 2-nitropropane, to obtain 115.0 g of 2-nitro-2-methyl-1-propanol crystals, and the gas chromatogram of the crystals is consistent with that of 2- The standard spectrogram of nitro-2-methyl-1-propanol is consistent, and its yield is 96.6% after detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com