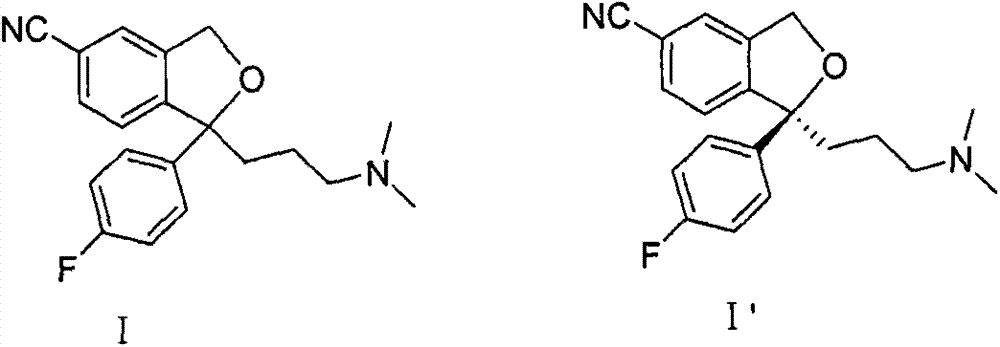
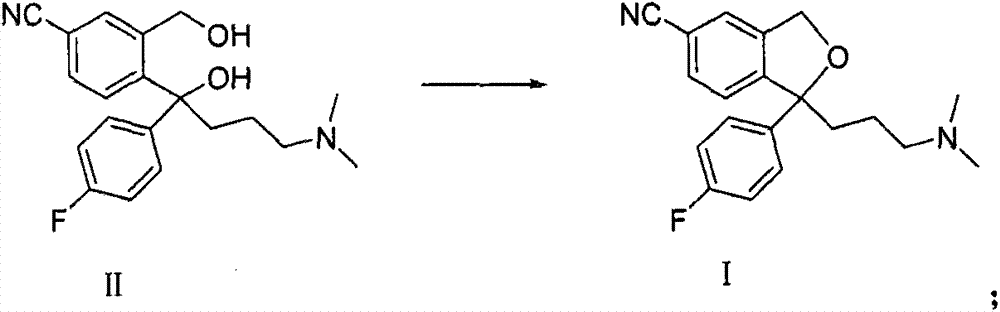
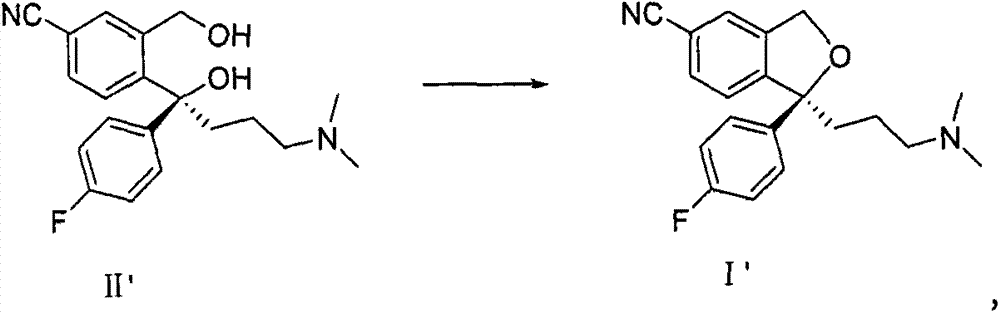
Method for preparing citalopram and S-citalopram
A C4-C7, organic solvent technology, applied in the direction of organic chemistry, can solve the problems of low solvent recovery rate, high production cost, large environmental pollution, etc., and achieve the effects of high solubility, increased production capacity, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 34.2 mol of diol compound (II) and 100 ml of methyl isobutyl ketone into a four-neck flask, stir to dissolve at room temperature, add 12 g of NaOH, add 50 ml of water, cool to 3°C, and then slowly dropwise add 100 ml of methyl alcohol A solution in which 27 g of p-toluenesulfonyl chloride was dissolved in isobutyl ketone was heated up to room temperature after the reaction was completed at 3°C. The layers were allowed to stand, and the aqueous layer was extracted once with 70ml of methyl isobutyl ketone; the combined organic layers were washed twice with 100ml of water each time. The yield of citalopram obtained by concentrating the organic layer was 83%, and the HPLC analysis result showed that the product had a purity of 99.6%.
Embodiment 2
[0027] Add 34.2mol S-diol compound (II') to the four-necked flask, add 100ml methyl isobutyl ketone, stir at room temperature to dissolve, add 12g NaOH, add 50ml water, cool to 3°C, and slowly add A solution of 27 g of p-toluenesulfonyl chloride was dissolved in 100 ml of methyl isobutyl ketone, and after the reaction was completed at 3° C., the temperature was raised to room temperature. The layers were allowed to stand, and the aqueous layer was extracted once with 70ml of methyl isobutyl ketone; the combined organic layers were washed twice with 100ml of water each time. The S-citalopram (I') obtained by concentrating the organic layer had a yield of 85%, and the HPLC analysis results showed that the product had a purity of 99.8%.
Embodiment 3
[0029] Add 34.2mol of diol compound (II) and 100ml of methyl isobutyl ketone into a four-necked flask, stir to dissolve at room temperature, add 12g of NaOH, add 50ml of water, cool to 3°C, and slowly add 100ml of A solution in which 16 g of methanesulfonyl chloride was dissolved in methyl isobutyl ketone was heated up to room temperature after the reaction was completed at 3°C. The layers were allowed to stand, and the aqueous layer was extracted once with 70ml of methyl isobutyl ketone; the combined organic layers were washed twice with 100ml of water each time. The yield of citalopram obtained by concentrating the organic layer was 80%, and HPLC analysis showed that the product had a purity of 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com