A photolysis reaction method of benzothiophene compounds for oxidative desulfurization
A technology of benzothiophene and compounds, which is applied in the field of oxidative photolysis reaction of benzothiophene compounds, can solve problems such as the difficulty of breaking C-S bonds, and achieve the effect of reducing the loss of hydrocarbons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
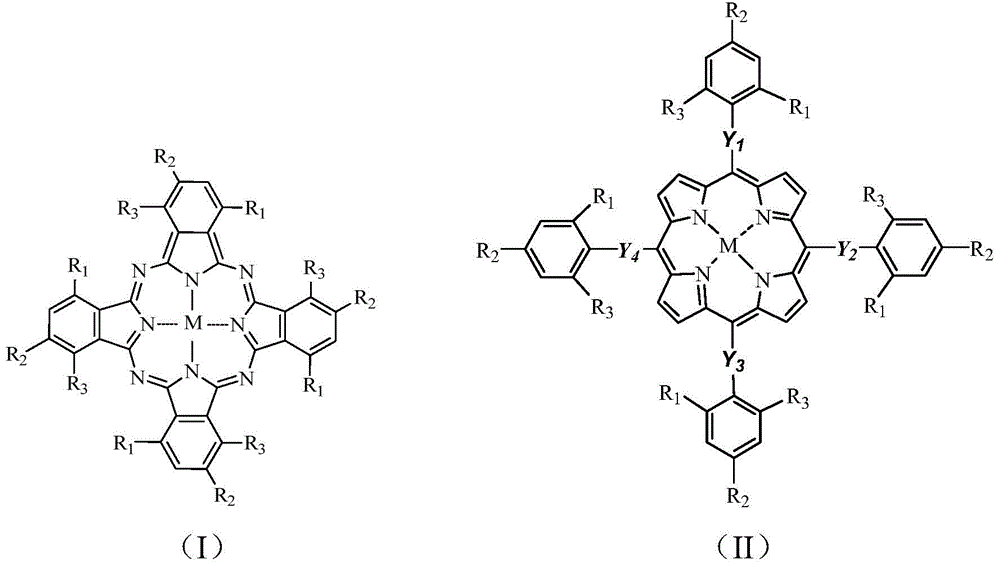
[0040] Weigh 30mg (0.15mmol) dibenzothiophene (DBT), 5mg o-chlorotetraphenylporphyrin iron (i.e. M=Fe in general formula (II), R 1 =Cl,R 2 =R 3 =H,Y 1 =Y 2 =Y 3 =Y 4 =C m , m=0), was dissolved in 30mL of methanol solution, slowly added dropwise 54mg (0.9mmol) of tert-butanol peroxide (TBHP) in the reaction mixture, and the mixture was stirred and reacted under the irradiation of a 250w high-pressure mercury lamp for 2.5 Hours, the reaction temperature was maintained at 36°C with cooling water circulation. The conversion rate of dibenzothiophene C-S bond breaking in the reaction product was 95.21% through chromatographic column separation and nuclear magnetic resonance detection.
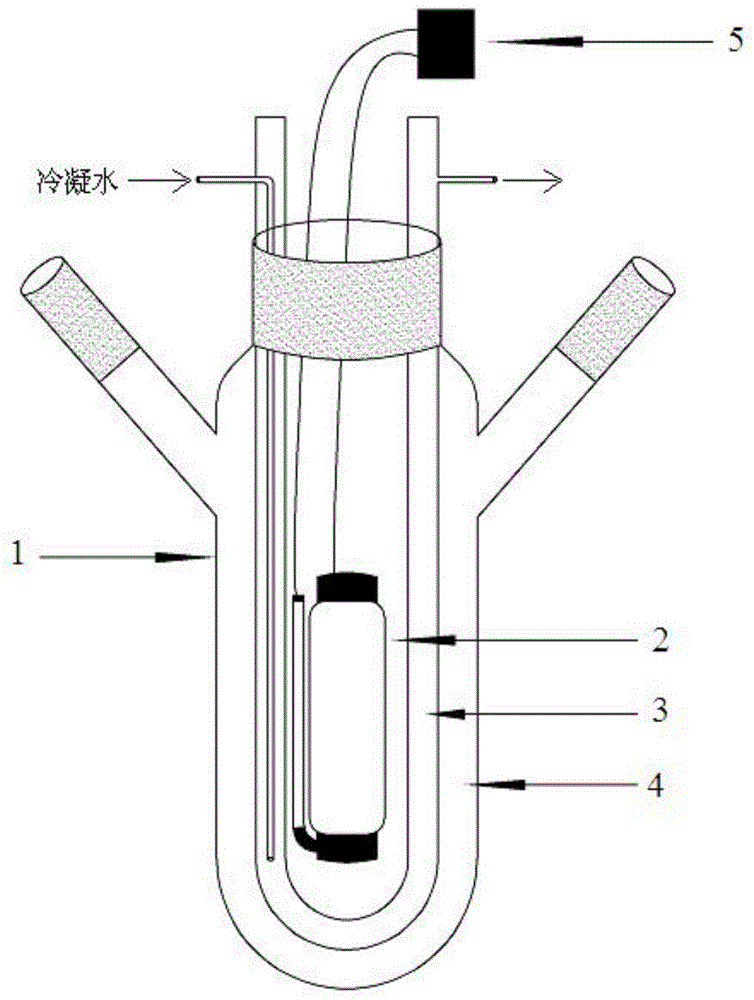
[0041] The above reaction preferably adopts such as figure 1 It is carried out in the reaction device shown, specifically: a three-port reactor 1, a U-shaped condenser tube 3 is installed from the central port, and a lamp 2 is installed in the U-shaped area of the condenser tube, and the la...
Embodiment 2
[0044] Weigh 30 mg of 4,6-dimethyldibenzothiophene (DMDBT), 5 mg of ferric o-chlorotetraphenylporphyrin, dissolve in 30 mL of methanol solution, slowly add 1.81 g of H 2 o 2 (30% aqueous solution) in the reaction mixture, the mixture was stirred and reacted for 2.5 hours under the irradiation of a 250w high-pressure mercury lamp, and the reaction temperature was kept at 36° C. with cooling water. Detected by high performance liquid chromatography, 100% conversion of 4,6-dimethyldibenzothiophene was detected, and the conversion rate of C-S bond breaking of 4,6-dimethyldibenzothiophene was 93.27% through chromatographic column separation and nuclear magnetic resonance detection %.
Embodiment 3
[0046] Take by weighing 30mg dibenzothiophene (DBT), 5mg iron phthalocyanine (being M=Fe in the general formula (I), R 1 =R 2 =R 3 =H,), dissolved in 30mL methanol solution, slowly added dropwise 0.72mg TBHP into the reaction mixture, the mixture was stirred and reacted for 2.5 hours under the irradiation of a 250w high-pressure mercury lamp, and the reaction temperature was kept at 25°C by circulating cooling water. Through chromatographic column separation and nuclear magnetic resonance detection, the conversion rate of dibenzothiophene C-S bond breaking in the reaction product is 67.20%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| decomposition efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com