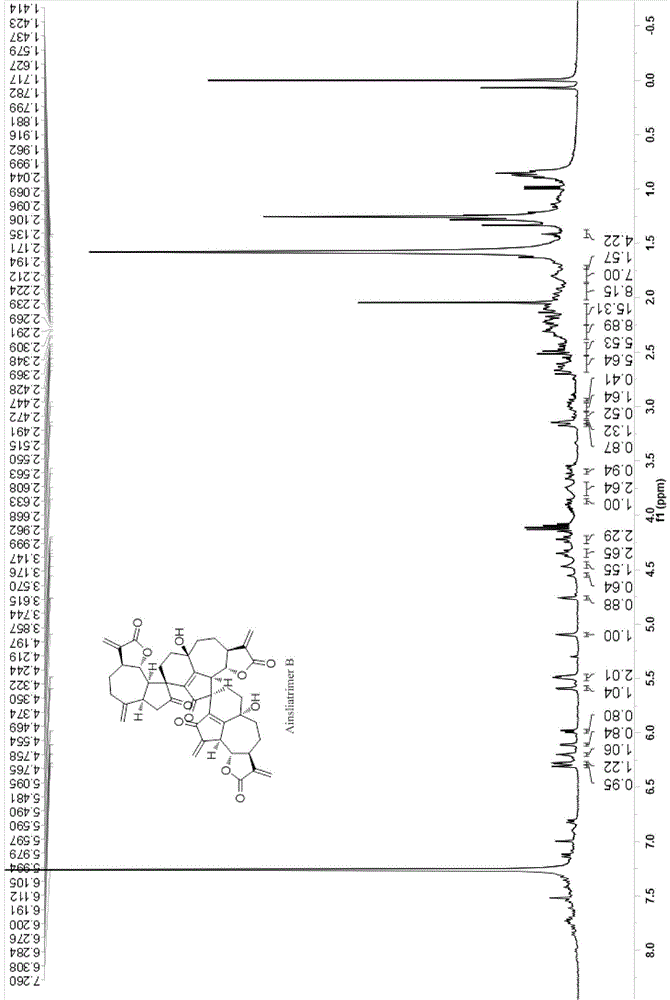
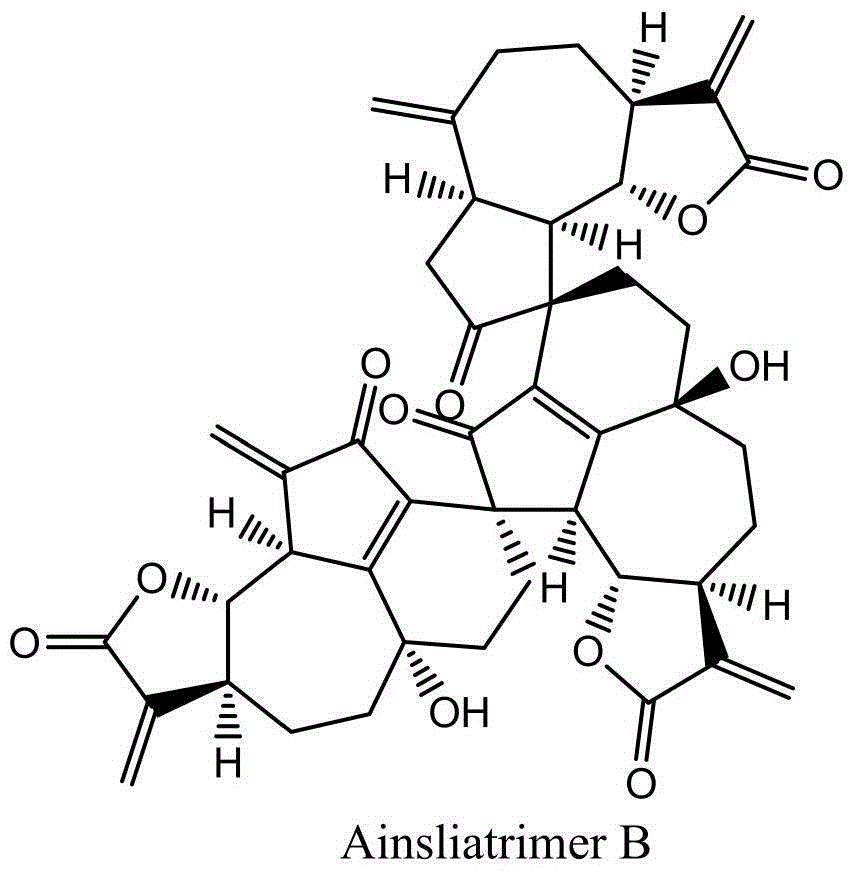
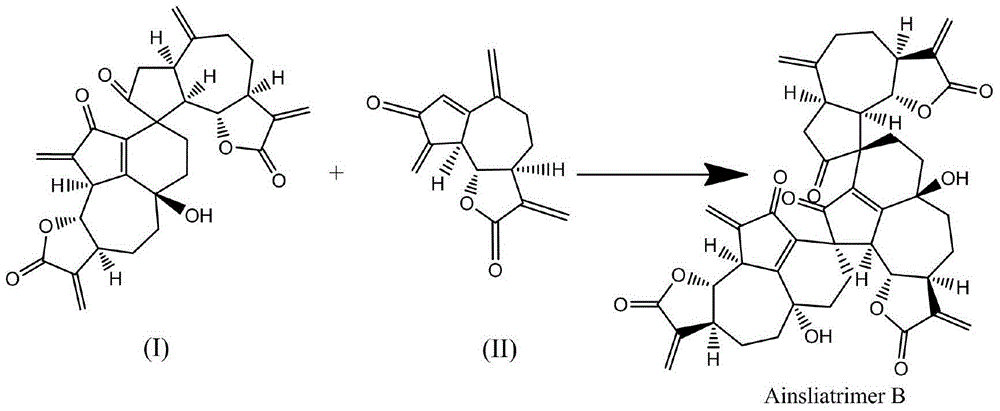
Ainsliatrimer B preparation method
A compound and reaction technology, which is applied in the field of preparation of ainsliatrimer B, can solve the problems of low yield, many steps in the synthesis method, and low yield, and achieve the effects of shortening the reaction steps, increasing the yield, and huge market value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] A preparation method of ainsliatrimer B, comprising the steps of:
[0037] Step 1, using dehydrocressin as a starting material to prepare the compound of formula (I), which specifically includes the following steps:
[0038] a. The compound of formula (III) is oxidized at the allylic position under the action of an oxidizing agent to prepare the compound of formula (IV);
[0039] b, making the compound of formula (IV) undergo an oxidation reaction under the action of an oxidizing agent to prepare a compound of formula (V);
[0040] c, making the compound of formula (V) react with iodotrimethylsilane to prepare the compound of formula (VI);
[0041] d, reacting the compound of formula (VI) with the compound of formula (IV), through a series reaction, including Saegusa oxidation, intermolecular Diels-Alder cycloaddition and free radical-mediated allylic oxidation reaction, to prepare formula (I) compounds;
[0042] The synthetic route is:
[0043]
[0044] In step ...
Embodiment 1
[0056] [Example 1] The chemical synthesis of ainsliatrimer B
[0057] 1. Preparation of isozaluzanin C (IV)
[0058] In a 2L three-necked flask, add 2.5g (10.86mmol) of dehydrocresinolide, dissolve it in 360mL of chloroform, slowly add 9.4mL (63.73mmol) of 65% tert-butanol hydrogen peroxide solution dropwise under stirring, and then add Selenium dioxide 282mg (2.54mmol), after reacting at room temperature for 8h, the reaction solution was filtered with silica gel, the filter residue was washed with 100mL dichloromethane, concentrated to 100mL by rotary evaporator, washed with 50mL10% sodium hydroxide solution and 50mL×2 brine The concentrated solution was dried over anhydrous sodium sulfate, filtered, the filter residue was washed with 20 mL of dichloromethane, the filtrate was combined, concentrated to dryness, and column chromatography (petroleum ether: ethyl acetate = 8:1-2:1) was recovered to obtain the raw material. 1.0130 g of hydrocressin to obtain isozaluzanin C (IV),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com