Preparation method of dry powder of recombinant human interferon-alpha-2b-BCG
A technology of recombinant human interferon and recombinant interferon, which is applied in the field of bioengineering, can solve the problems of poor process controllability, easy pollution, and low rate of viable bacteria, and achieve large single-time production, reduce pollution, and increase the activity of bacteria. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
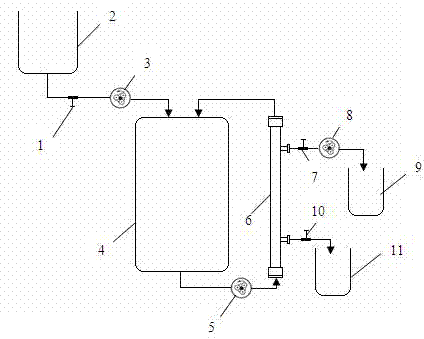
[0033] The present invention will be described in detail below in conjunction with the accompanying drawings and embodiments.
[0034] 1.Main materials and equipment
[0035] 1. Middlebrook (Middlebrook) 7H9 medium is on sale
[0036] Attached table 1 Middlebrook 7H9 medium composition
[0037]
[0038] 2. The fermenter adopts the American NBS BIOFLO415 fermenter with a working volume of 5 liters.
[0039] 3. Tangential flow hollow filter column 1.5PD600, produced by GE Healthcare, USA.
[0040] 4. Peristaltic pump, China Baoding Lange Constant Flow Pump Co., Ltd., BT600-2J, 0.07-3000 ml / min;
[0041] Peristaltic pump for NBS BIOFLO415 fermenter.
[0042] 2. Product preparation
[0043] 1. Seed activation and preparation
[0044] Freeze or cryopreserve the liquid culture recovery of recombinant interferon-α-2b-BCG seed batches, and inoculate the strains into 250 mL of freshly prepared Middlebrook 7H9 liquid medium containing 30 μl / ml kanamycin, and keep the temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com