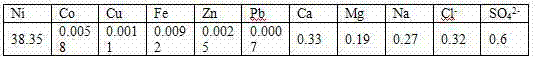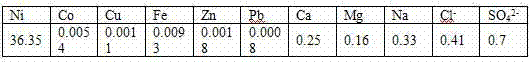Nickel fluoride preparation method
A technology of nickel fluoride and ammonium fluoride, applied in the direction of nickel halide, etc., which can solve the problems of strong fluorine gas, toxicity, and high equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] A kind of preparation method of nickel fluoride, its process is successively:
[0017] a. Slurry of nickel carbonate
[0018] Slurry the nickel carbonate with pure water according to the liquid-solid ratio of 3:1-4:1;
[0019] b. Synthetic preparation
[0020] Raise the temperature of the slurried nickel carbonate to 75°C-90°C, add ammonium fluoride according to 1.25-1.4 times the mass of nickel carbonate to ensure excess ammonium fluoride, keep the temperature at 75°C-90°C, and react under stirring conditions for 2 -3 hours, ammonia gas is produced during the reaction;
[0021] c. Purify and remove impurities
[0022] A large amount of ammonia gas will be produced during the reaction process, but due to the high solubility of ammonia gas, there is not much ammonia gas volatilized. This part of ammonia gas dissolved in the solution will form nickel ammonia complexes with nickel ions, resulting in the formation of nickel fluoride Redissolution, so after the reaction ...
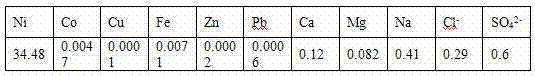
Embodiment 1
[0026] a. Slurry of nickel carbonate
[0027] The nickel carbonate is slurried according to the liquid-solid ratio of nickel carbonate to pure water of 3:1, and then the temperature is raised to 75°C.
[0028] b. Synthetic preparation
[0029] Add ammonium fluoride to the slurry formed in step a for synthetic preparation, the amount of ammonium fluoride added is 1.25 times the mass of nickel carbonate, keep the temperature at 75°C, and react for 2 hours under stirring conditions, during the reaction process Ammonia gas is produced.
[0030] c. Purify and remove impurities
[0031] Add anhydrous sodium carbonate to the substance formed in step b, the specific amount added is 0.3 times the amount of the nickel carbonate substance, keep the temperature at 75°C, and react for 2 hours.
[0032] d. Washing, filtering and drying
[0033] After the reaction, according to the liquid-solid ratio of 3:1, the filter cake was washed twice with pure water, and then the filter cake was d...
Embodiment 2
[0037] a. Slurry of nickel carbonate
[0038] The nickel carbonate is slurried according to the liquid-solid ratio of nickel carbonate to pure water of 4:1, and then the temperature is raised to 80°C.
[0039] b. Synthetic preparation
[0040] Add ammonium fluoride to the slurry formed in step a for synthetic preparation, the amount of ammonium fluoride added is 1.3 times the mass of nickel carbonate, keep the temperature at 80°C, and react for 2 hours under stirring conditions, during the reaction process Ammonia gas is produced.
[0041] c. Purify and remove impurities
[0042] Add potassium carbonate to the substance formed in step b, the specific amount added is 0.3 times the amount of nickel carbonate substance, keep the temperature at 80°C, and react for 1.5 hours.
[0043] d. Washing, filtering and drying
[0044] After the reaction, according to the liquid-solid ratio of 2:1, the filter cake was washed twice with pure water, and then the filter cake was dried until...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com