Fluorescent quantum dot used for magnetic resonance contrast agent and preparation method thereof
A fluorescent quantum dot, quantum dot technology, applied in nuclear magnetic resonance/magnetic resonance imaging contrast agents, chemical instruments and methods, luminescent materials, etc., can solve problems such as unpublished literature reports, and achieve good monodispersity, Simple operation, good cytocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
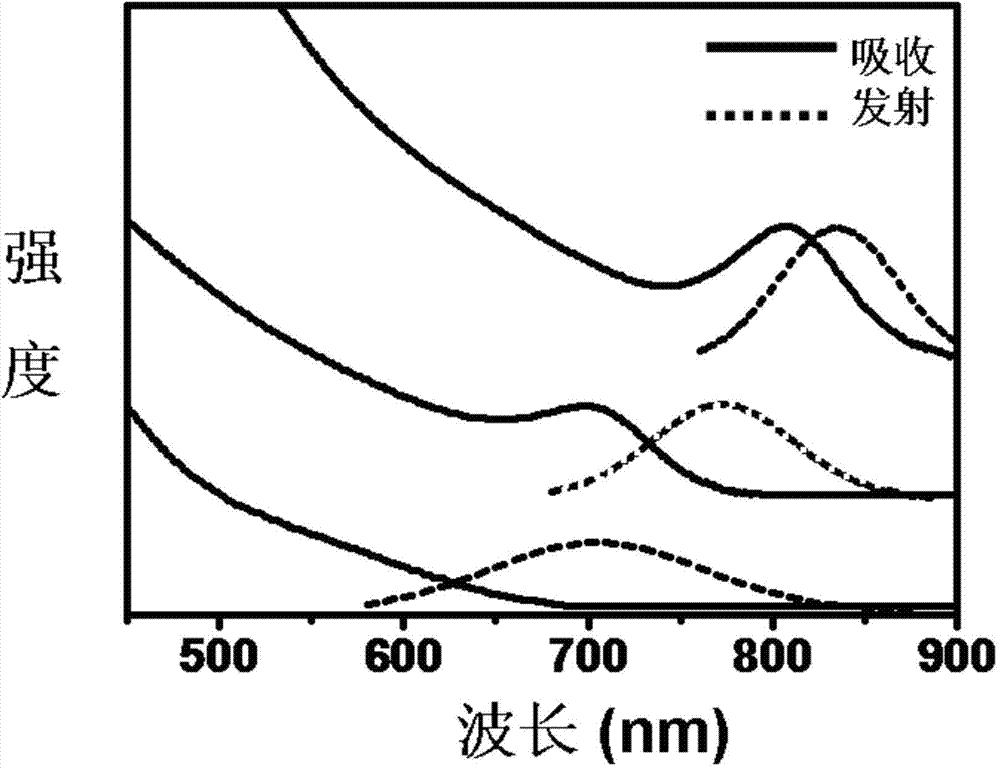
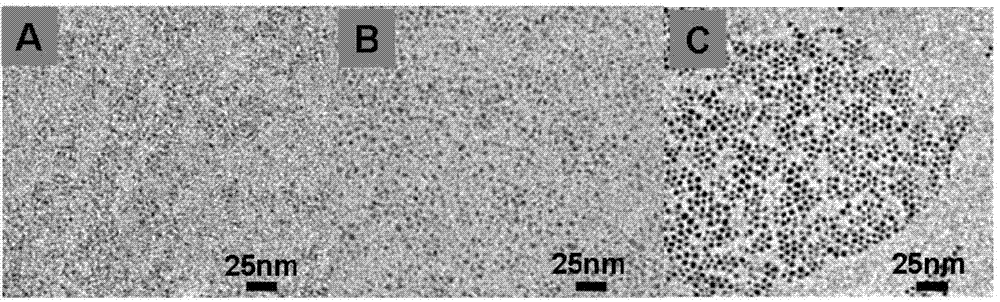
[0025] First, 5ml of octadecene, 0.1mmol of silver acetate and 0.01mmol of gadolinium chloride, 0.1mmol of oleic acid and 0.1mmol of hexaalkylmercaptan were added to the reaction flask, then the solution was heated to 50°C, and 0.05mmol of elemental sulfur was dissolved in 0.05ml Hexaalkylthiol and quickly injected into the above reaction solution and maintained for 20 minutes to obtain GdAg with a particle size of 1.5 nm and a stoichiometric ratio of Gd:Ag of 1:10 2 S quantum dots.
[0026] Do not change the GdAg in this embodiment 2 S quantum dot preparation raw materials and preparation process, only the solution is heated to 50 ° C to change the solution temperature to 75 ° C, and the stoichiometric ratio of Gd: Ag is 1: 10 to obtain GdAg with a particle size of 2.6 nm. 2 S quantum dots.
[0027] Do not change the GdAg in this embodiment 2 S quantum dot preparation raw materials and preparation process, only the solution is heated up to 50 ° C to change the solution tem...
Embodiment 2
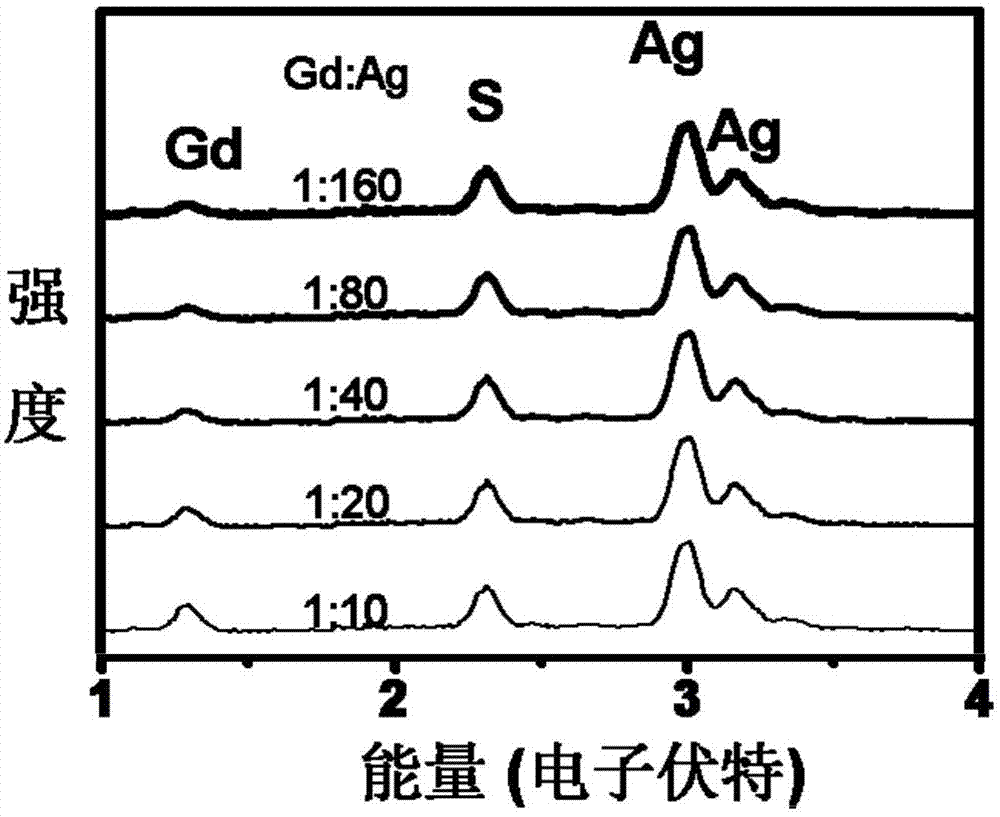
[0031] First, 50ml of octadecene, 1mmol of silver acetate and 0.00625mmol of gadolinium chloride, 1mmol of oleic acid and 1mmol of hexaalkyl mercaptan were added to the reaction flask, then the solution was heated to 75°C, and 0.5mmol of elemental sulfur was dissolved in 0.5ml of hexaalkylsulfur Alcohol and quickly injected into the above reaction solution and kept for 20 minutes to prepare GdAg with a particle size of 2.6 nm and a stoichiometric ratio of Gd:Ag of 1:160 2 S quantum dots.
[0032] Do not change the GdAg in this embodiment 2 S quantum dot preparation raw materials and preparation process, only the amount of gadolinium chloride was changed to 0.00625 × 2mmol, 0.00625 × 4mmol, 0.00625 × 8mmol, 0.00625 × 16mmol respectively, and the stoichiometric ratio Gd:Ag can be obtained respectively 1:160, 1:80, 1:40, 1:20, 1:10 GdAg with a particle size of 2.6nm 2 S quantum dots.
[0033] image 3 It is the elemental analysis result of quantum dots with a particle size of...
Embodiment 3
[0035]First, 20ml of octadecene, 0.4mmol of silver acetate, 0.04mmol of gadolinium chloride, 0.4mmol of oleic acid and 0.4mmol of hexaalkylmercaptan were added to the reaction flask, then the solution was heated to 120°C, and 0.2mmol of elemental sulfur was dissolved in 0.2ml of hexaalkylene mercaptan. In the alkylthiol and quickly injected into the above reaction solution and kept for 20 minutes, the GdAg with a stoichiometric ratio of Gd:Ag of 1:10 and a size of 3.5 nm was obtained. 2 S quantum dots.
[0036] Figure 4 It is the X-ray powder diffraction analysis result of the quantum dots with a particle size of 3.5nm and a stoichiometric ratio (Gd:Ag:S=0.1:1:0.5) prepared in Example 3 at a reaction temperature of 120°C.
[0037] Do not change the GdAg in this embodiment 2 S quantum dot preparation raw materials and preparation process, only change the amount of gadolinium chloride to 0.01mmol, and finally can prepare GdAg with a stoichiometric ratio of Gd:Ag of 1:40 and a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com