Biological preparation and preparation method for preventing and controlling human respiratory syncytial virus infection
A technology of biological agents and syncytial virus, applied in antiviral agents, respiratory diseases, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
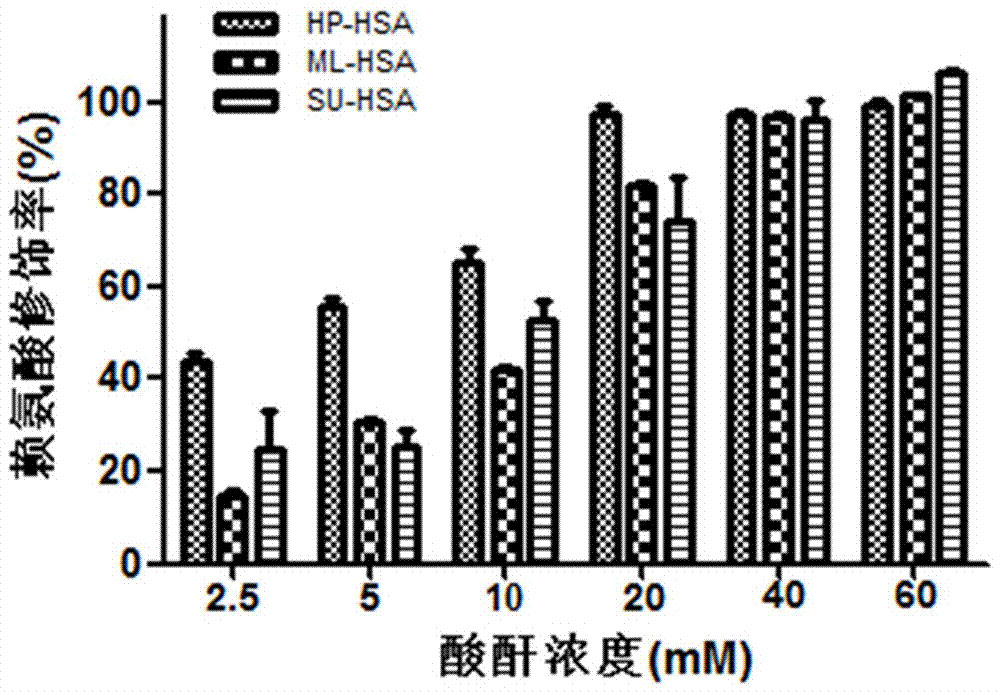
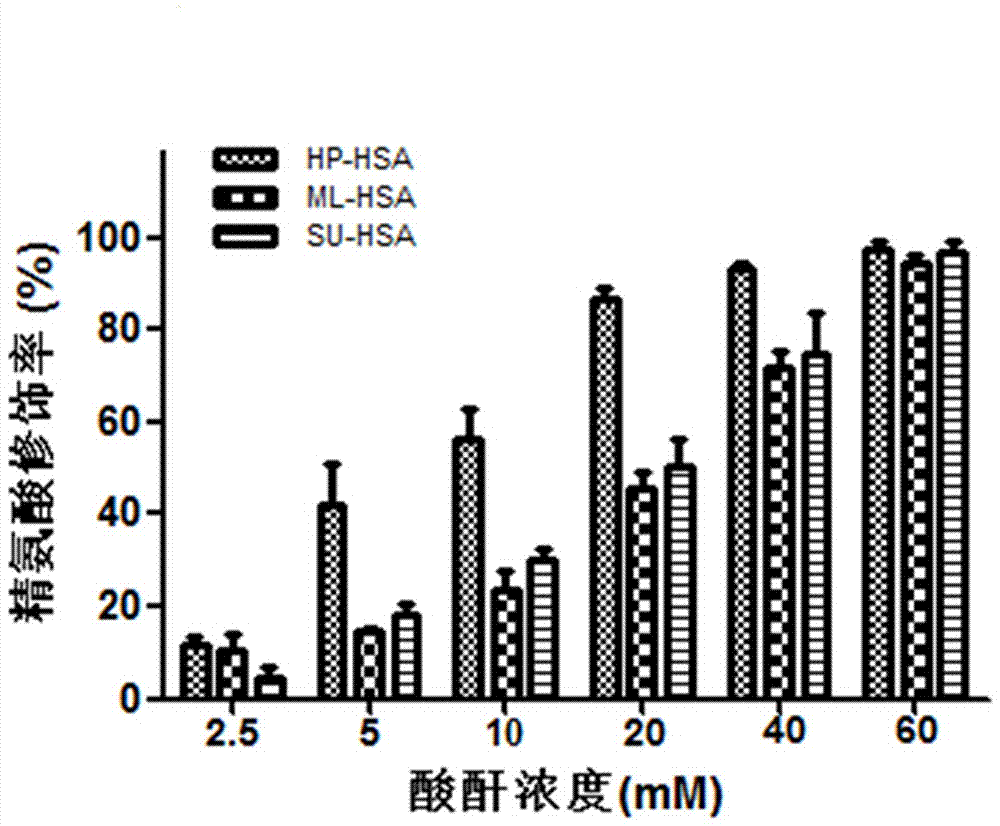
[0082] A preparation method of a biological preparation for preventing and controlling human respiratory syncytial virus infection, taking HSA as an example, purchasing human serum albumin; using active acid anhydride to acid anhydride the purchased protein, and repurifying the active protein after dialysis; including the following steps :
[0083] (1) Dissolve maleic anhydride (ML) in DMSO to a saturated concentration;
[0084] (2) Human serum albumin (HSA) was dissolved in 0.1M sodium phosphate solution at pH 8.5 to obtain a protein solution with a final protein concentration of 20 mg / mL;
[0085] (3) Add the ML solution to the protein solution several times, mix well after each addition, and adjust the pH to 8.5, the final concentration of the acid anhydride in the reaction system is 60mM;
[0086] (4) Under the condition of temperature of 25°C, let stand for 1 hour, then dialyze the PBS solution with pH 7.4 at 4°C. Then use a 0.45 μM microporous membrane to filter and st...
experiment example 2
[0089] A preparation method of a biological agent for preventing and controlling human respiratory syncytial virus infection, comprising the following steps:
[0090] (1) Dissolving derivatives of maleic anhydride in DMSO to obtain a saturated anhydride solution;
[0091] (2) Dissolve chicken serum albumin OVA in 0.1 M sodium phosphate solution at pH 8 to obtain a protein solution with a final protein concentration of 20 mg / mL;
[0092] (3) Divide the above ML solution into five equal parts, add it to the protein solution every 12 minutes, mix well, adjust the pH to 8 with 1M NaOH, and the final concentration of acid anhydride in the reaction system is 40mM;
[0093] (4) Under the condition of temperature of 20°C, let it stand for 1 hour, then dialyze the PBS solution with pH 7 at 4°C overnight. Then use a 0.45 μM microporous membrane to filter and sterilize, store at 4°C, and then make ointment according to the existing method.
[0094] In the biological preparation prepare...
experiment example 3
[0096] A preparation method of a biological agent for preventing and controlling human respiratory syncytial virus infection, comprising the following steps:
[0097] (1) Dissolve maleic anhydride (ML) in DMSO to a saturated concentration;
[0098] (2) Dissolve the stable protein mainly isolated and purified β-lactoglobulin (β-LG) from milk products in 0.1 M sodium phosphate solution at pH 9 to obtain a protein solution with a final protein concentration of 20 mg / mL ;
[0099] (3) Divide the above ML solution into five equal parts, add it to the protein solution every 12 minutes, mix well, adjust the pH to 9 with 1M NaOH, and the final concentration of acid anhydride in the reaction system is 50mM;
[0100] (4) Under the condition of temperature of 30°C, let it stand for 1 hour, then dialyze the PBS solution with pH 8 at 4°C overnight. Then use a 0.45 μM microporous membrane to filter and sterilize, store at 4°C, and then make a gel according to the existing method.
[0101...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com