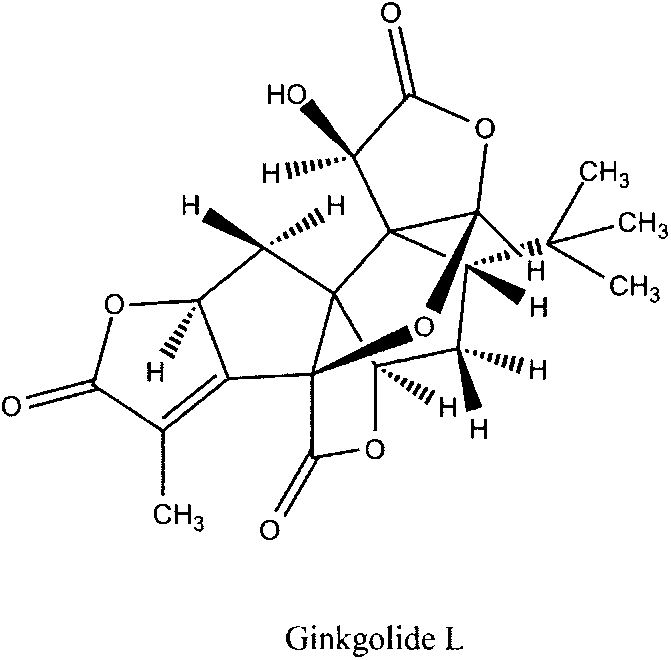
Preparation method of ginkgolide L
A technology of ginkgolides and total ginkgolides, which is applied in the field of medicine and can solve the problem of low content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
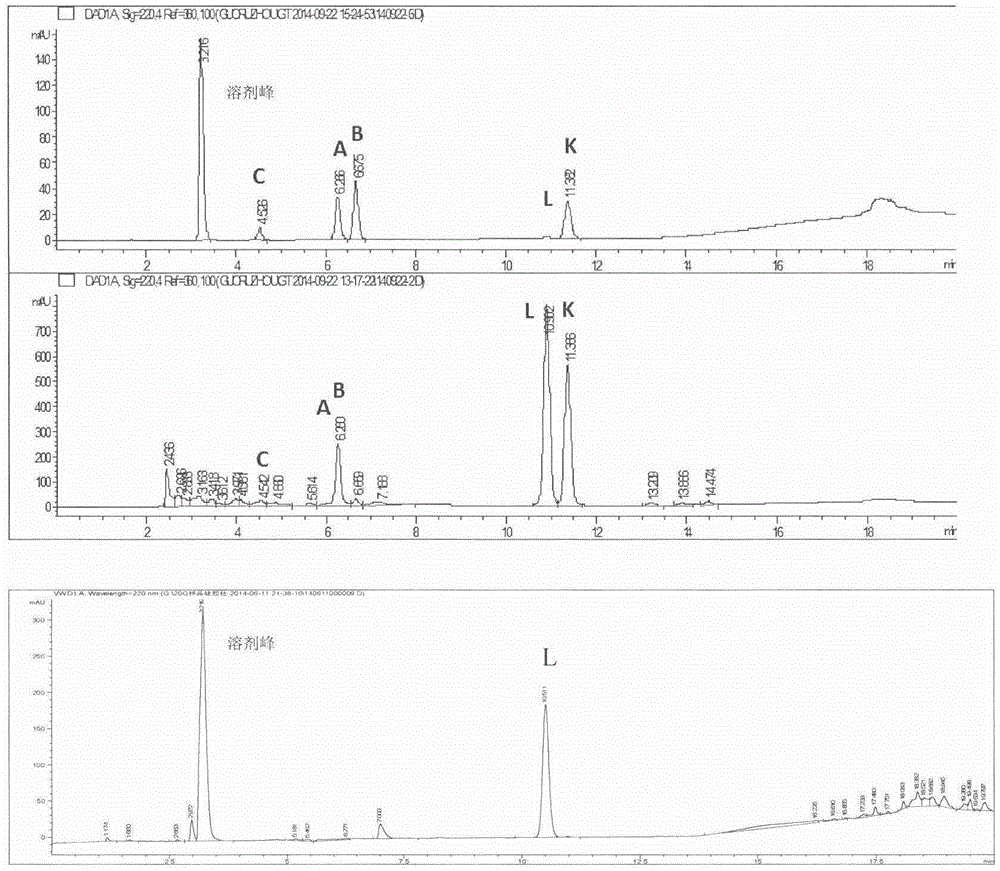
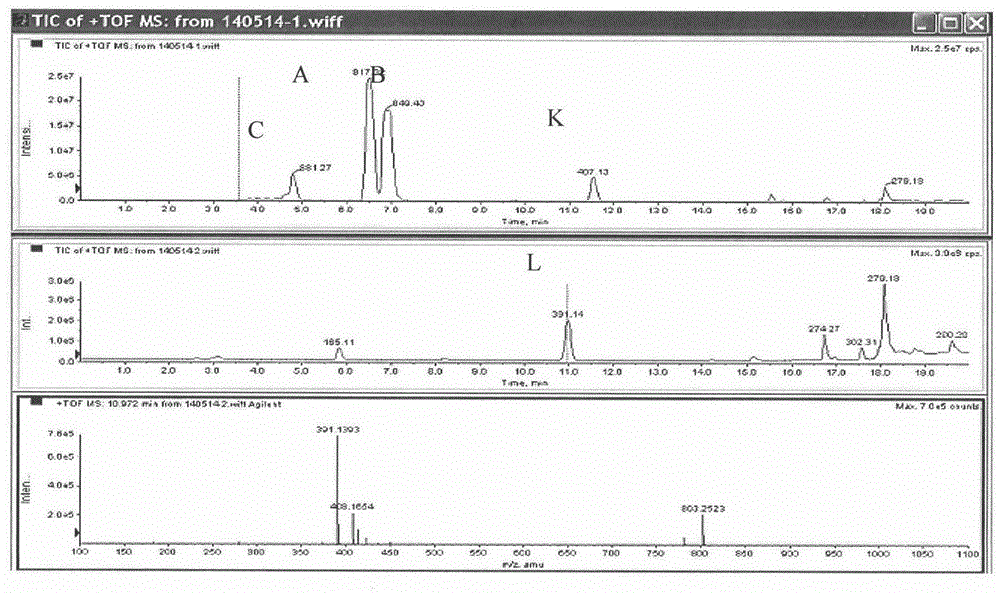
[0066] Weigh 50 mg of total ginkgo lactones, dissolve in 100 ml of methanol solution, and ultrasonically promote dissolution. Heat the oil bath to 70°C, slowly add 1ml of concentrated sulfuric acid dropwise, raise the temperature to 140°C, and react until the solution turns light yellow and transparent, about 6 hours. Cooled in an ice bath, and stood overnight in a refrigerator at 4°C to precipitate a solid, which was filtered. Choose 100-mesh silica gel, dry-pack the column, dry-load the sample, choose chloroform-methanol as the eluent, and perform isocratic elution, and the elution gradient is according to 100:1--80:1--50:1--10: 1--1:1 decrease, collect the eluate, use HPLC-TOF to detect the eluate components, after detection, it is clear that the eluate contains ginkgolide L, and spin dry to obtain ginkgolide L. The yield is about 40%.
Embodiment 2
[0068] Weigh 50 mg of total ginkgo lactones, dissolve in 100 ml of acetone-water solution (ratio: 5:1), and ultrasonically promote dissolution. Heat the oil bath to 80°C, add 2ml of 37% concentrated hydrochloric acid, condense and reflux, raise the temperature to 130°C, and react for 4 hours. Standing in an ice bath for crystallization, a brown solid was obtained. Choose 300 mesh silica gel, pack in wet method, mix the brown solid into diatomaceous earth according to the weight ratio of 1:1, load the sample in dry method, use petroleum ether-ethyl acetate as the eluent, and perform isocratic elution with an elution gradient of 200 : 1--100: 1--50: 1--10: 1--1: 1 Decrease, collect the eluate, use HPLC-TOF to detect the components of the eluate, after detection, it is clear that the content of ginkgolide L The eluate was spin-dried to obtain ginkgolide L. The yield is about 38%.
Embodiment 3
[0070] Weigh 20 mg of total ginkgo lactones, dissolve in 100 ml of isopropanol-water solution (ratio: 10:1), and sonicate to promote dissolution. Heat the oil bath to 75°C, slowly drop 3-4 drops of acetic anhydride, condense and reflux, raise the temperature to 135°C, and react for 6 hours. Standing in an ice bath for crystallization, a brown solid was obtained. Use 300-mesh silica gel, dry-pack the column, mix the brown solid into diatomaceous earth according to the weight ratio of 1:1, dry-load the sample, use petroleum ether-acetone as the eluent, and use isocratic elution with an elution gradient of 300:1 --200:1--100:1--10:1--1:1 Decrease, collect the eluate, use HPLC-TOF to detect the composition of the eluate, after detection, it is clear that the elution containing ginkgolide L solution, spin-dried to obtain ginkgolide L. The yield is about 37%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com