Indol-pyrrolidine -2-ketone alkaloid and preparation method and application thereof
A technology of tetrahydropyrrole and alkaloid, applied in the biological field, can solve the problems of low oxidation degree, single compound structure, reporting and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The preparation method of indole-tetrahydropyrrol-2-one alkaloids of the present invention comprises the following steps:
[0031] A. Solid fermentation: culture the strain in potato dextrose agar medium at 28°C for 7 days, inoculate it into a 50-500ml Erlenmeyer flask containing 10-100ml of potato dextrose medium, and rotate and shake at 180rpm at 28°C Under the conditions of shaking culture for 5-10 days to obtain the nutrient medium containing bacteria, inoculate it for large-scale solid fermentation to obtain fermentation products;
[0032] B. Extraction of extract: add an organic solvent with a solid-to-liquid volume ratio of 1.5-3 times to the fermentation product and extract by cold soaking for 2-4 times, each time for 30-60 minutes, combine the extracts, filter, and concentrate under reduced pressure to 1 / 4~ 1 / 2 volume, let it stand, filter out the precipitate, concentrate (evaporate to dry ethyl acetate) to obtain the extract;
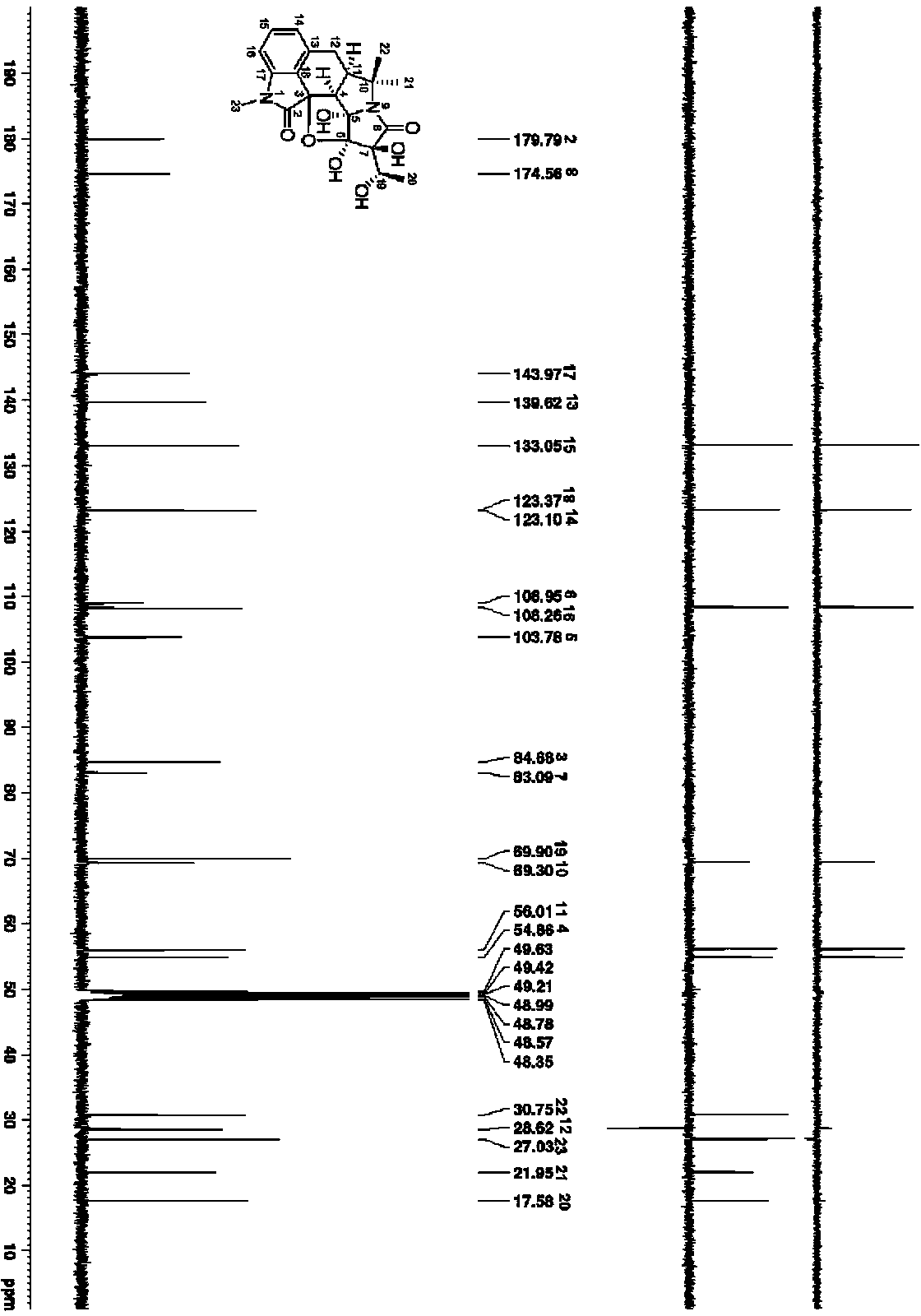
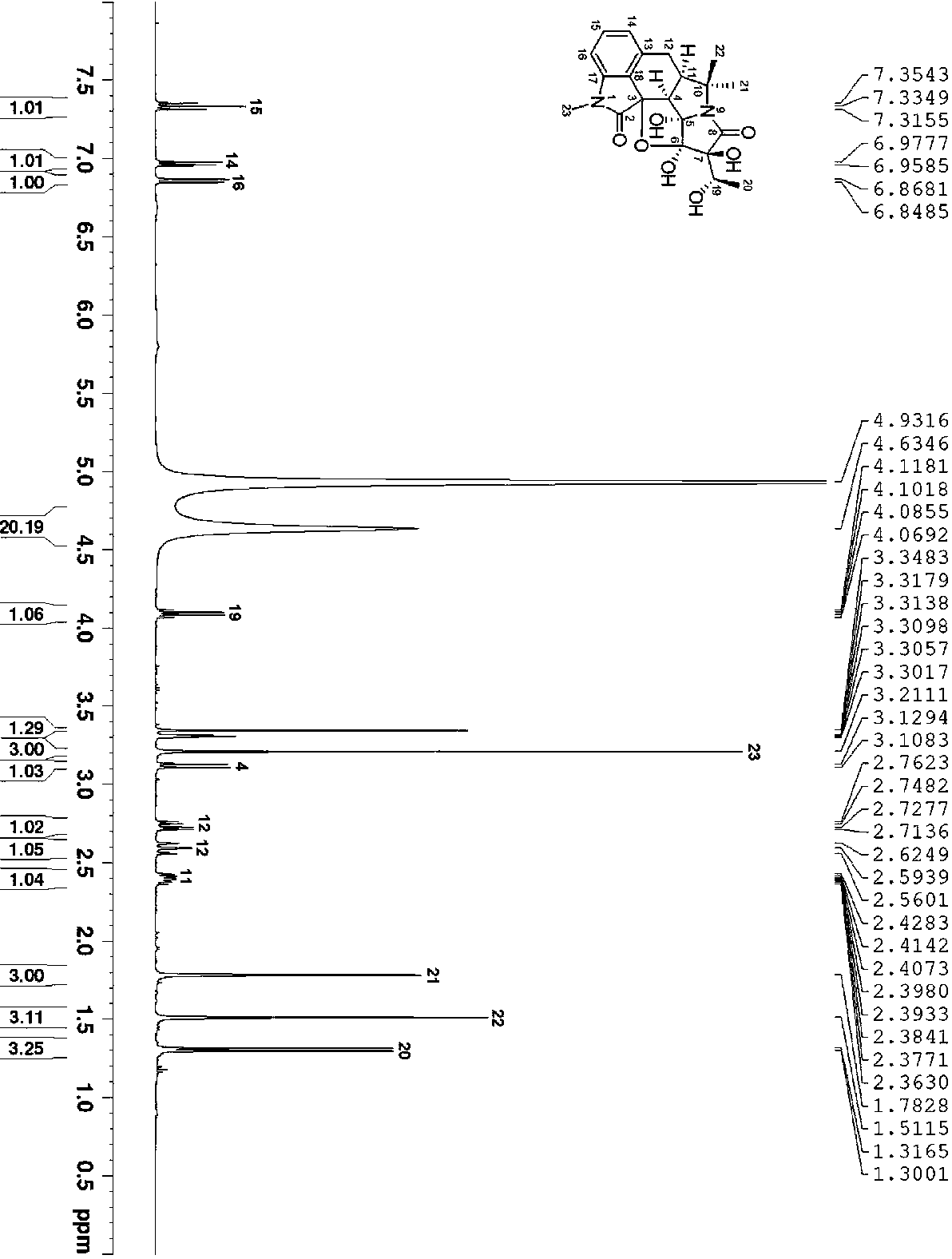
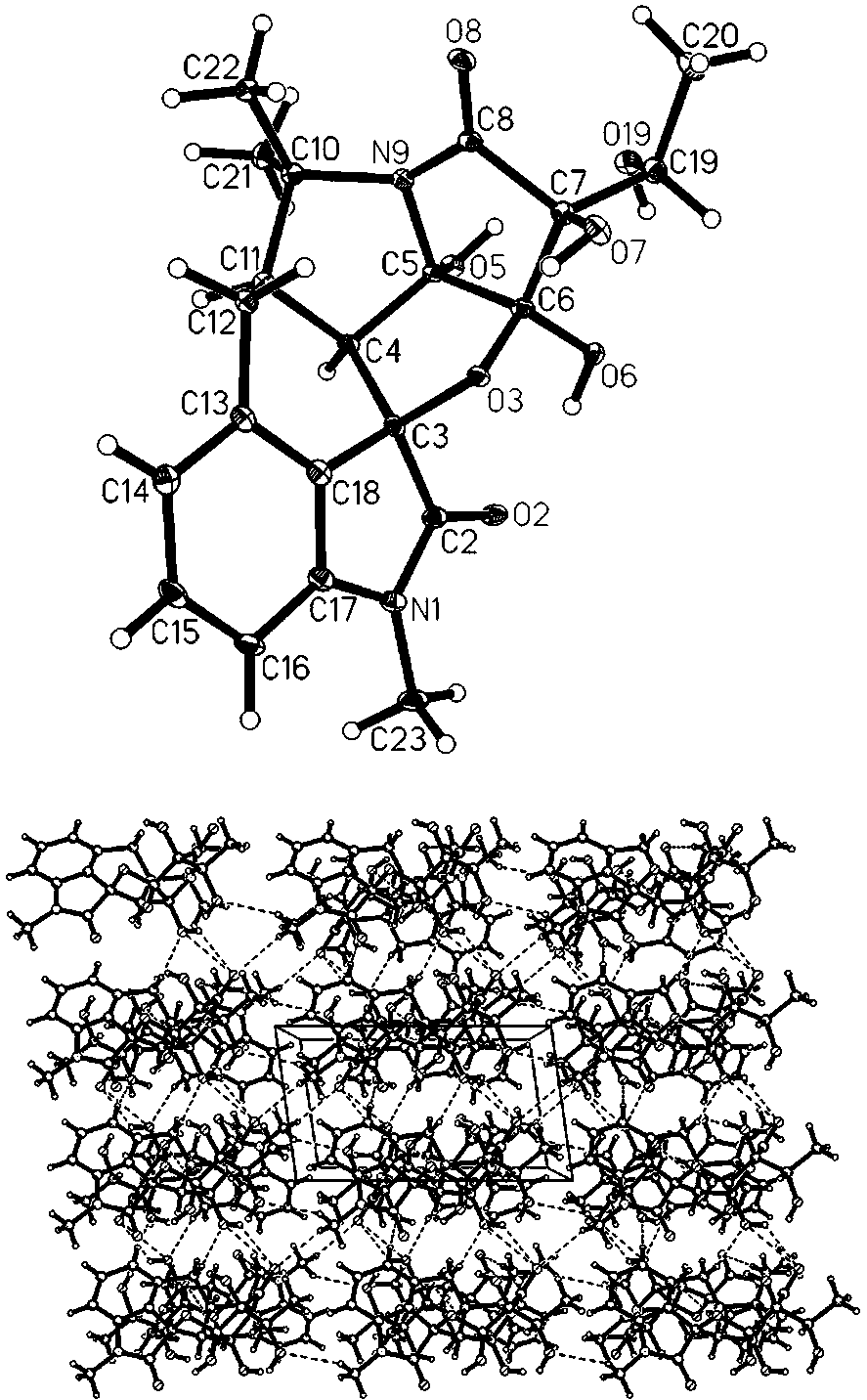
[0033]C. Silica gel column chro...
Embodiment 1
[0078] A. Solid fermentation and extract extraction:
[0079] The Penicillium clade Penicillium group ( Penicillium ramigena, YNCA0361) bacterial strain was inoculated on the potato dextrose agar medium at room temperature, cultivated for 7 days at 28 degrees Celsius, inoculated in 250 milliliters of conical flasks, each conical flask contained 100 milliliters of potato dextrose medium, and placed in 28 degrees Celsius for shaking culture for 5 day (180 rpm). The large-scale fermentation takes place in 200 500ml vonbach bottles, each containing 100g of rice and 120ml of distilled water. Each bottle was inoculated with 5.0 ml of bacteria-containing nutrient medium, cultured at 25 degrees Celsius for 45 days, extracted by cold soaking in acetone 4 times, each time for 30-60 minutes, and the extracts were combined; the extracts were filtered, and the extracts were concentrated under reduced pressure to 1 / 4 ~ When 1 / 2 the volume, let it stand still, filter out the precipitate, a...
Embodiment 2
[0083] A. Solid fermentation and extract extraction:
[0084] The Penicillium clade Penicillium group ( Penicillium ramigena,YNCA0361) strains were inoculated on potato dextrose agar medium at room temperature, and cultivated at 28 degrees Celsius for 10 days. Inoculate in 500 ml Erlenmeyer flasks, each Erlenmeyer flask contains 200 ml potato dextrose medium, place at 28 degrees Celsius for 7 days (180 rpm). Large-scale fermentation was carried out in 100 250ml vonbach bottles, each containing 50g of rice and 60ml of distilled water. Each bottle was inoculated with 2.5 milliliters of bacteria-containing nutrient medium, and cultured at 25 degrees Celsius for 30 days. Extract by cold soaking with organic solvent 4 times, each time for 30~60min, combine the extracts; filter the extract, concentrate the extract under reduced pressure to 1 / 4~1 / 2 volume, let stand, filter out the precipitate, and concentrate to 300g Extract a;
[0085] B. Compound separation
[0086] The extra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ic50 value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com