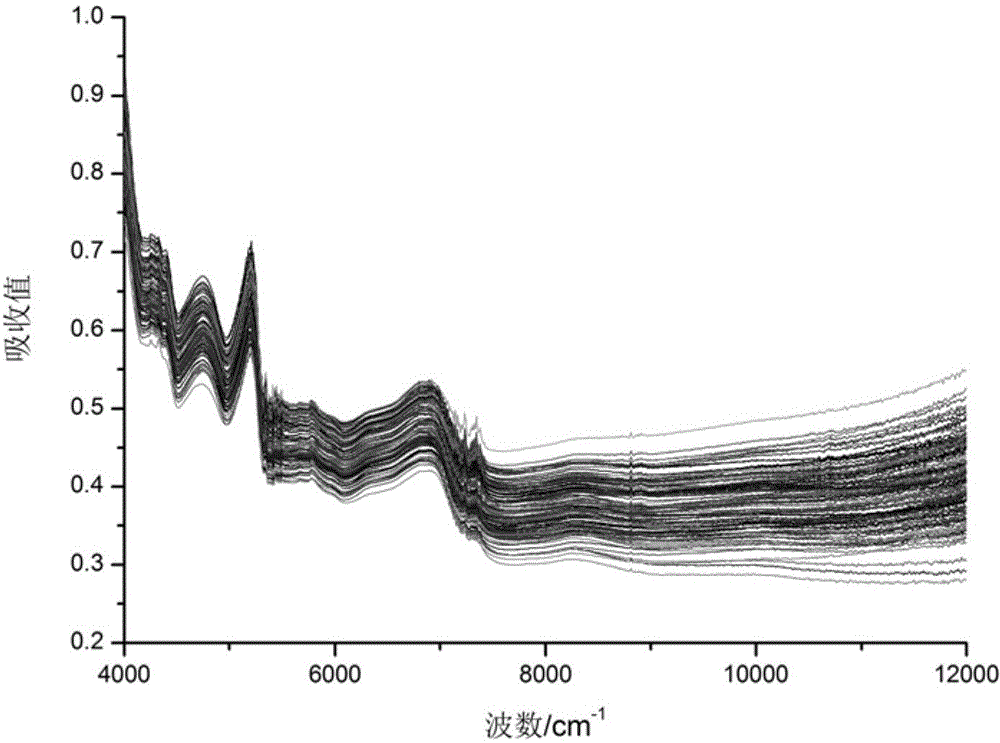
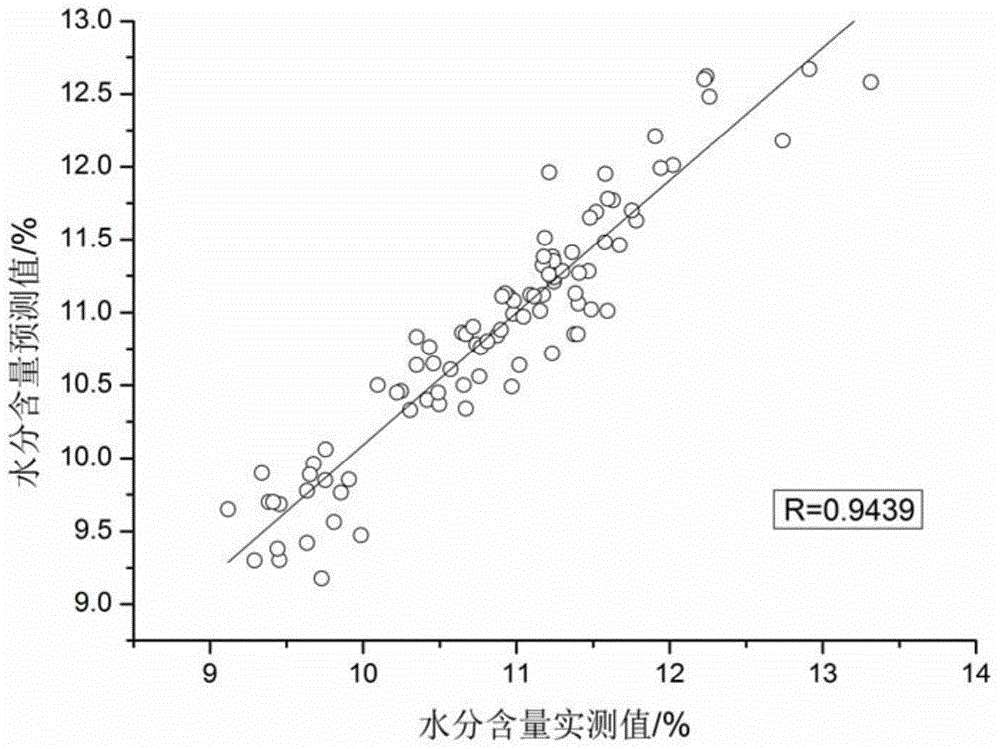
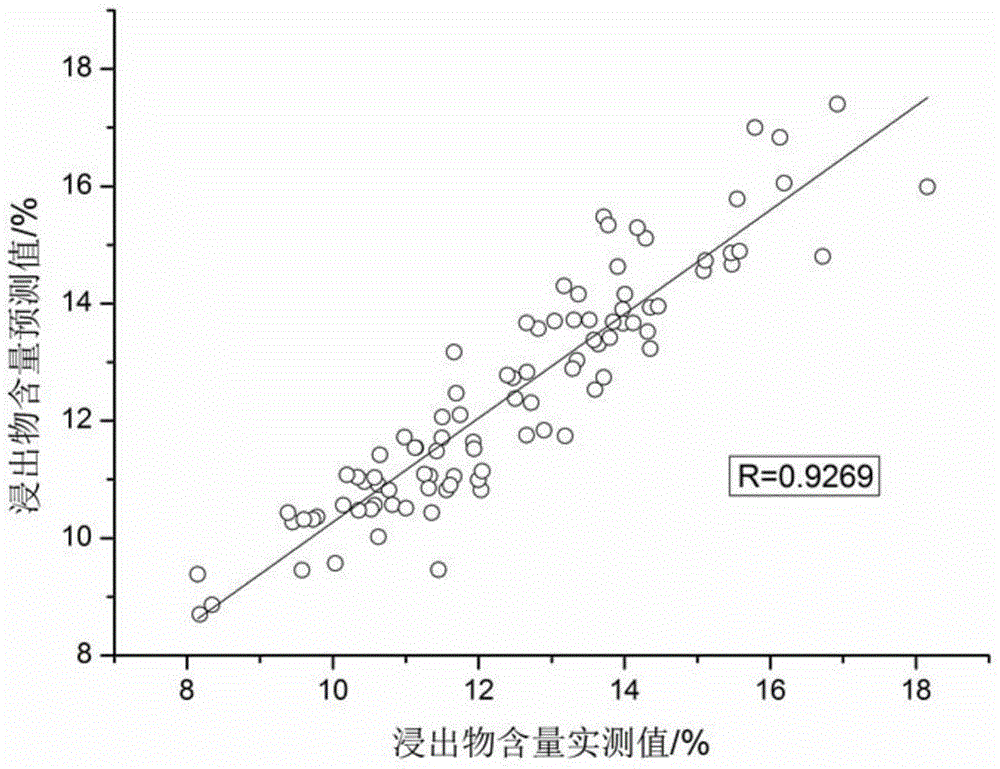
Method for quickly detecting ramulus uncariae cum uncis by utilizing near-infrared spectrometry and application of method
A near-infrared spectroscopy and near-infrared spectroscopy technology, applied in the field of medical testing, can solve the problems of not being able to adapt to the modern development of traditional Chinese medicine preparations, not reflecting the complexity of Chinese medicine ingredients, and difficult production practices, so as to improve production efficiency and economic benefits, shorten The effect of high detection time and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] A. Determination of moisture content includes the following steps:
[0059] (1) After selecting a total of 110 parts of Uncaria medicinal materials from different origins with known moisture content and pulverizing them, pass through an 80-mesh sieve to obtain Uncaria medicinal material powder with a relatively uniform particle size for subsequent use; wherein the Uncaria medicinal material powder is dried according to Method is used to measure the moisture content of the described Uncaria medicinal material, and obtain the standard content of the moisture of the described Uncaria medicinal material, the specific steps are as follows:
[0060] The moisture determination of Uncaria medicinal material is according to the drying weighing method recorded in the 2010 edition of "Chinese Pharmacopoeia", and the flat bottle (X 0 ), get the described Uncaria medicinal material powder of 2g, accurately weigh (X 1 ), baked in a vacuum oven at 105°C for 5 hours, took it out and c...
Embodiment 2
[0071] B. The determination of extract content includes the following steps:
[0072] (1) A total of 110 parts of Uncaria medicinal materials from different origins with known extract content were selected and pulverized, and passed through an 80-mesh sieve to obtain Uncaria medicinal material powder with a relatively uniform particle size, which was set aside; wherein the Uncaria medicinal material powder was heated according to Determination of the content of the extract of the Uncaria medicinal material by immersion method to obtain the standard content of the water extract of the Uncaria medicinal material, the specific steps are as follows:
[0073]Get described Uncaria medical material powder 1g, accurately weighed (X 1 ), placed in a 100mL Erlenmeyer flask, precisely added 25mL of ethanol, sealed tightly, weighed, allowed to stand for 1h and then ultrasonically extracted for 1h. After cooling down, weigh again, make up for the lost weight with ethanol, shake well, plac...
Embodiment 3
[0084] C, the mensuration of rhynchophylline content comprises the following steps:
[0085] (1) After selecting a total of 110 parts of Uncaria medicinal materials from different origins with known moisture content and pulverizing them, pass through an 80-mesh sieve to obtain Uncaria medicinal powder with a relatively uniform particle size, and set aside; The content of the rhynchophylline of the described Uncaria medicinal material is determined by phase chromatography, and the standard content of the rhynchophylline of the described Uncaria medicinal material is obtained, and the specific steps are as follows:
[0086] a. The preprocessing method is:
[0087] Take about 2 g of the Uncaria medicinal material powder, weigh it accurately, add 25 mL of methanol with a mass concentration of 75% precisely, put it in a refrigerator at 4°C for 2 hours, extract it ultrasonically for 60 minutes, use 75% methanol to make up for the weight loss, and then transfer the extract Centrifug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com