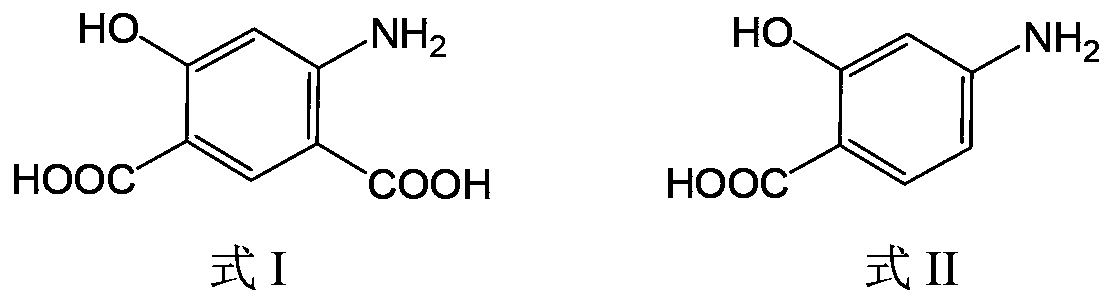Method for industrial production of isophthalic acid derivatives
A technology of potassium formate and potassium carbonate, applied in chemical instruments and methods, preparation of organic compounds, preparation of cyanide reactions, etc., can solve problems such as limiting production efficiency and scale, consuming energy, being uneconomical, etc., and achieving cost saving and shortening. Response time, simple effect of production equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
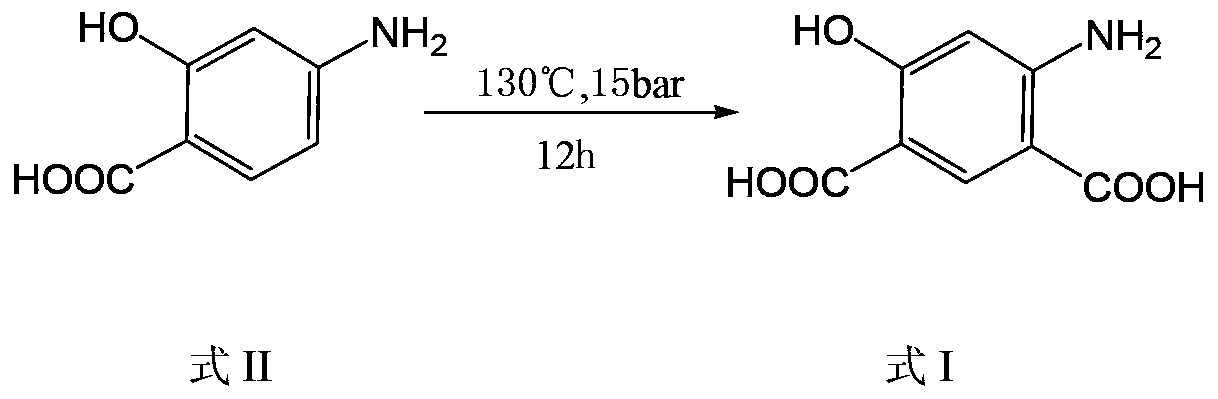
[0037] In a three-necked reaction flask, add 30.6g of p-aminosalicylic acid, 27.6g of potassium carbonate, and 184.9g of potassium formate, mix well, pass CO2 gas replacement twice, continue to pass CO2 under 1 bar pressure, and heat the reaction system At 190-195°C, the potassium formate melts completely, and reacts at this temperature for 2.5 hours. After the reaction is completed, add DMSO to the reaction system, stir to form a slurry, then add water and ethyl acetate for extraction and layering, discard the organic phase, adjust the water phase to acidity with 1M dilute hydrochloric acid, precipitate solids, filter, and dry to obtain 38.0 g of 4-amino-6-hydroxy-isophthalic acid.
Embodiment 2
[0039] In a three-necked reaction flask, add 25.0g of p-aminosalicylic acid, 33.8g of potassium carbonate, 8.1g of potassium bicarbonate, and 26.9g of potassium formate. CO2, heat the reaction system to 185-190°C, the potassium formate is completely melted, and react at this temperature for 3.5 hours. After the reaction is completed, add DMSO to the reaction system, stir to form a slurry, then add water and ethyl acetate for extraction and layering, discard the organic phase, adjust the water phase to acidity with 1M dilute hydrochloric acid, precipitate solids, filter, and dry , 31.0 g of 4-amino-6-hydroxy-isophthalic acid was obtained.
Embodiment 3
[0041] In a three-necked reaction flask, add 20.1g of p-aminosalicylic acid, 35.9g of potassium carbonate, 13.8g of potassium bicarbonate, and 65.9g of potassium formate. CO2, heat the reaction system to 171-178°C, the potassium formate is completely melted, and react at this temperature for 1 hour. After the reaction is completed, add DMSO to the reaction system, stir to form a slurry, then add water and ethyl acetate for extraction and layering, discard the organic phase, adjust the water phase to acidity with 1M dilute hydrochloric acid, precipitate solids, filter, and dry , 24.0 g of 4-amino-6-hydroxy-isophthalic acid was obtained. Example 4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com