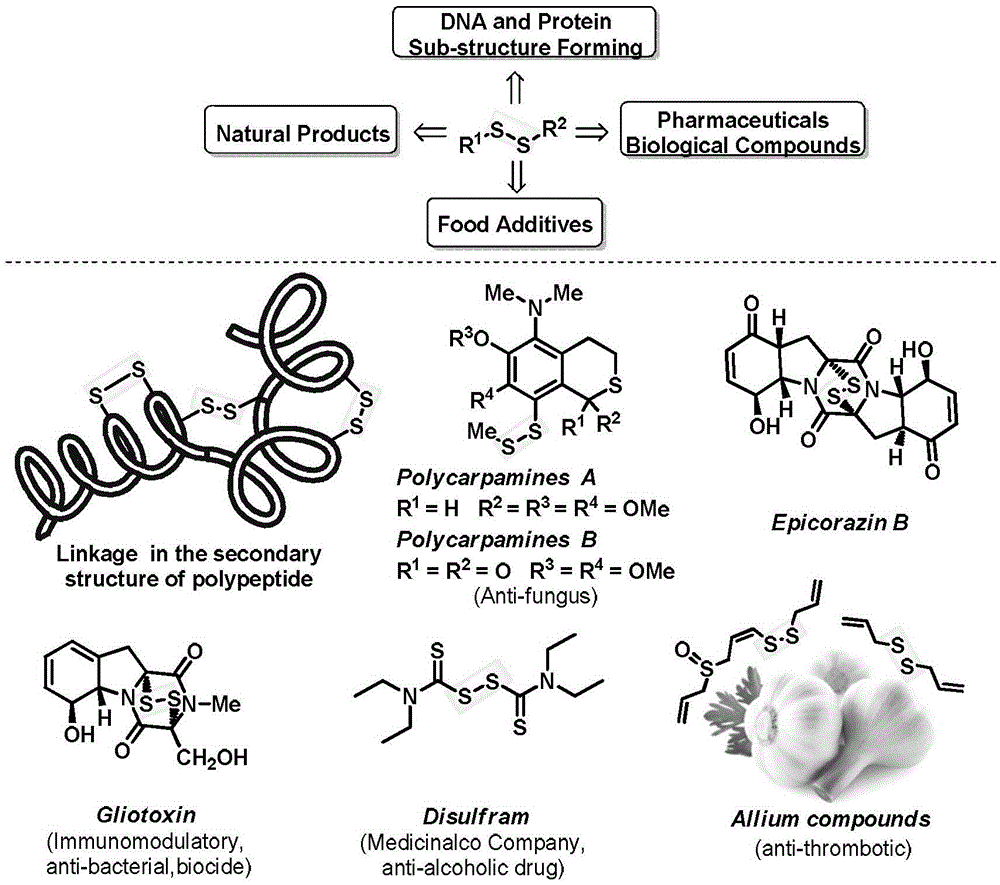
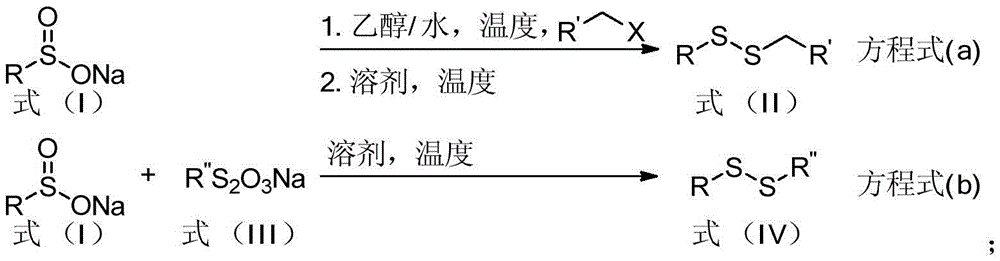
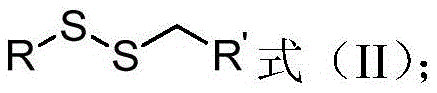Aryl-aryl, aryl-alkyl, alkyl-alkyl asymmetric persulfide compound and synthesis method thereof
A synthesis method and asymmetric technology, applied in the field of organic compound process application, can solve the problems of irritating odor, easy oxidation of sulfurized reagents, expensive catalyst, etc., and achieve the effects of no irritating odor, efficient reaction and mild conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Synthesis of 4-(4-methylphenyldithio)butyronitrile:
[0046]
[0047] 4-Chlorobutyronitrile (103.5mg, 1.0mmol, 5.0equiv.) and Na 2 S 2 o 3 ·5H 2 O (248.2mg, 1.0mmol, 5.0equiv.) was added to the reaction tube, and then the reaction solvent ethanol / water (0.25mL / 0.5mL) was added, and stirred at 100°C for 2 hours. Then the reaction system was cooled, and the solvent was removed under reduced pressure, then sodium p-toluenesulfinate (35.6 mg, 0.2 mmol, 1.0 equiv.) was added, and then the reaction solvent 1,4-dioxane (1.0 mL ), and stirred at reflux for 11 hours at a reaction temperature of 110°C. Then the reaction system was cooled to room temperature, and PPh was added 3 (31.5mg, 0.12mmol, 0.6equiv.) was reacted at 50°C for 4 hours, then the reaction solution was decompressed to remove the solvent, and the product 1 was obtained after separation by column chromatography (eluent polarity: petroleum ether / ethyl acetate ester 40:1). Yield: 94%; 1 HNMR (400MHz, CDCl...
Embodiment 2
[0049] Synthesis of 4-(4-methylphenyldithio)butyronitrile:
[0050]
[0051] 4-Chlorobutyronitrile (103.5mg, 1.0mmol, 5.0equiv.) and Na 2 S 2 o 3 ·5H 2 O (248.2mg, 1.0mmol, 5.0equiv.) was added to the reaction tube, and then the reaction solvent ethanol / water (0.25mL / 0.5mL) was added, and stirred at 100°C for 2 hours. Then the reaction system was cooled, and the solvent was removed under reduced pressure, then sodium p-toluenesulfinate (35.6 mg, 0.2 mmol, 1.0 equiv.) was added, and then the reaction solvent 1,4-dioxane (1.0 mL ), stirred at a reaction temperature of 90° C. for 11 hours. Then the reaction system was cooled to room temperature, and finally the solvent was removed from the reaction solution under reduced pressure, and the product 1 was obtained after separation by column chromatography (eluent polarity: petroleum ether / ethyl acetate 40:1). Yield: 72%;
Embodiment 3
[0053] Synthesis of 4-(4-methylphenyldithio)butyronitrile:
[0054]
[0055] 4-Chlorobutyronitrile (103.5mg, 1.0mmol, 5.0equiv.) and Na 2 S 2 o 3 ·5H 2 O (248.2mg, 1.0mmol, 5.0equiv.) was added to the reaction tube, and then the reaction solvent ethanol / water (0.25mL / 0.5mL) was added, and stirred at 100°C for 2 hours. Then the reaction system was cooled, and the solvent was removed under reduced pressure, then sodium p-toluenesulfinate (35.6 mg, 0.2 mmol, 1.0 equiv.) was added, and then the reaction solvent 1,4-dioxane (1.0 mL ), stirred at a reaction temperature of 70° C. for 11 hours. Then the reaction system was cooled to room temperature, and finally the solvent was removed from the reaction solution under reduced pressure, and the product 1 was obtained after separation by column chromatography (eluent polarity: petroleum ether / ethyl acetate 40:1). Yield: 37%;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com