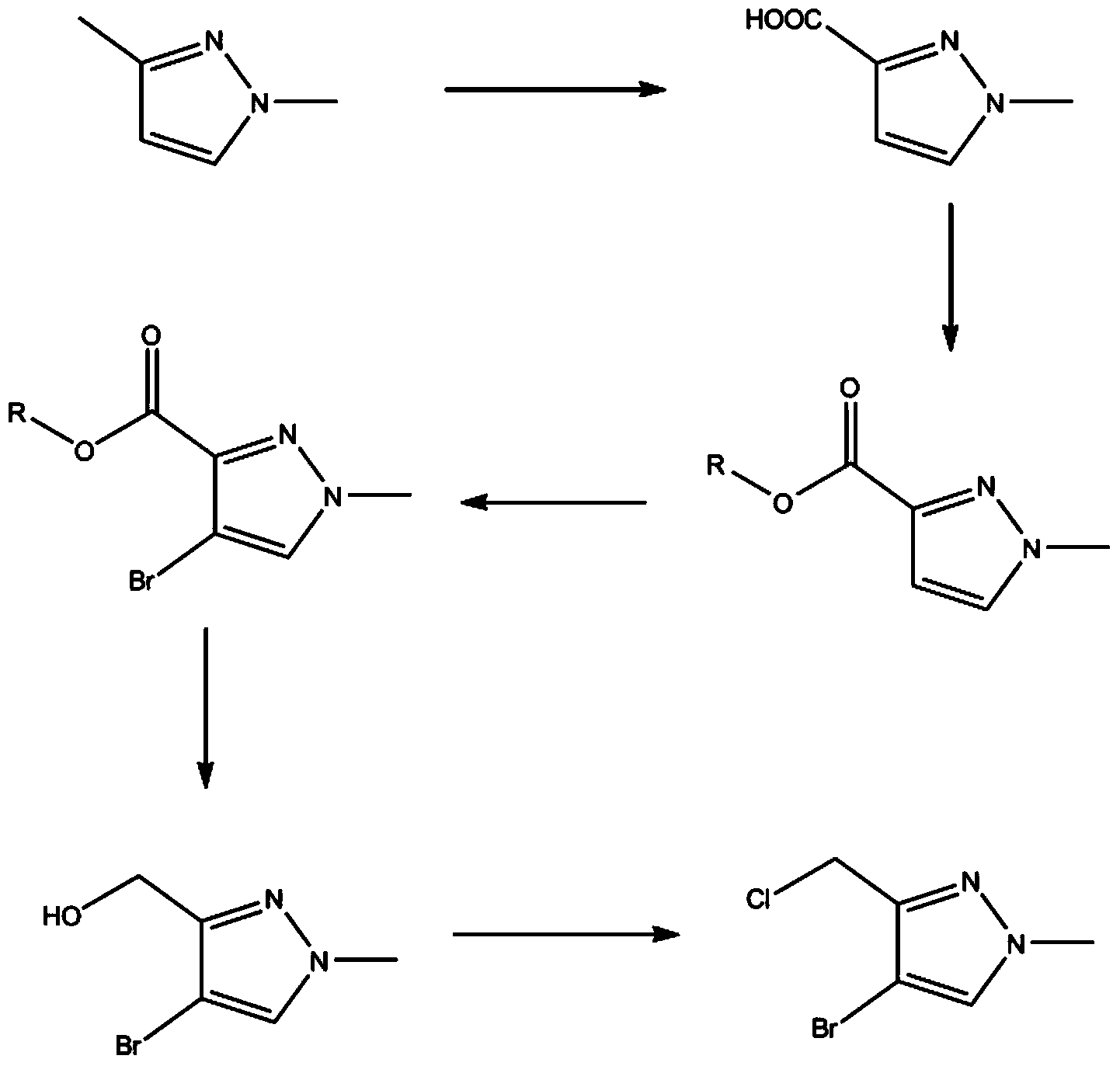
Method for preparing 4-bromo-3-chloromethyl-1-methyl-1H-pyrazole
A technology of dimethylpyrazole and chloromethyl, applied in the field of organic compound synthesis, can solve the problems of difficult purification, unsuitable for industrial production, and many side reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
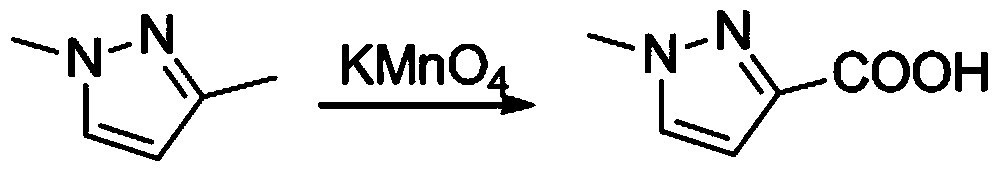
[0070] Example 1. Oxidation of 1,3-dimethylpyrazole to 1-methyl-3-carboxy-pyrazole
[0071] (1), the reaction equation is as follows:
[0072]
[0073] (2), specific process steps:
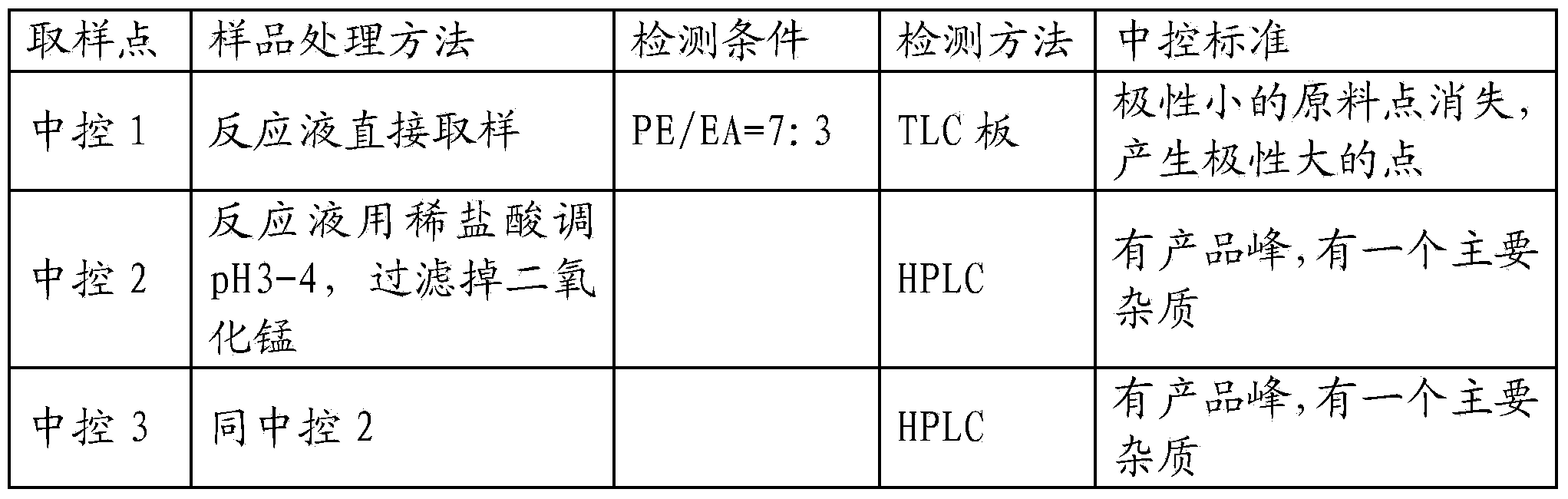
[0074] Dissolve 160g of potassium permanganate in 1L of water and place it in a 5L four-neck flask, one port is connected to a mechanical stirrer, one port is connected to a dropping funnel, and the other port is covered with three consecutive buffer balls to prevent material flushing, the temperature is raised to 40, and slowly Add 100g of 1,3-dimethylpyrazole (dissolved in 500mL water) dropwise, unplug the heating mantle when the temperature of the reaction solution rises to 60 degrees, and the temperature of the reaction solution will rise to 70- 80 degrees, control the rate of addition to ensure that the reaction temperature is within 80 degrees to avoid flushing (stop dripping when it rises to 80 degrees), and the dripping is completed in 3 hours (central control 1). After dropping, add ...
Embodiment 2
[0081] Example 2. Esterification of 1-methyl-3-carboxy-pyrazole into 1-methyl-3-ester-pyrazole
[0082] (1), the reaction equation is as follows:
[0083]
[0084] (2), specific process steps:
[0085] Put 180 g of pulverized 1-methylpyrazole-3-carboxylic acid in a 500 mL four-necked flask, add 360 g of toluene, drop 5 drops of N, N'-dimethylformamide (DMF), stir, and the reaction is White turbid liquid, when the temperature rises to 75°C, start to add thionyl chloride dropwise. The dropping process absorbs heat, but outgasses violently. Control the dropping speed to avoid flushing. After the dropwise addition of thionyl chloride is completed, keep stirring at 75°C for 30 minutes. , the reaction solution becomes clear, then add anhydrous methanol dropwise at this temperature, release gas violently, control the rate of addition, reflux for 30 minutes after the addition of methanol is completed, take a sample for detection (in the control 1) and then cool the reaction soluti...
Embodiment 3
[0090] Example 3. Halogenation of 1-methyl-3-ester-pyrazole to 4-bromo-1-methyl-3-ester-pyrazole
[0091] (1), the reaction equation is as follows:
[0092]
[0093] (2), specific process steps:
[0094] Add 140g of methyl 1-methylpyrazole-3-carboxylate and 320ml of dichloromethane into the reaction flask, protect it with nitrogen, stir and clarify, and cool the reaction flask in an ice bath. When the internal temperature is 10°C, add dibromosea in batches Because of 157.3g, the addition process released heat, and the solution turned yellow and turbid. The speed of adding dibromohydantoin was controlled so that the reaction temperature was not higher than 15°C. After the addition, TLC was performed (in the control 1). No raw materials, post-processing, slowly add the reaction solution into 300g (350mL, 400mL, 450mL, 500mL) water, stir for 30min, separate layers, use 200g of 10% sodium sulfite solution for the organic layer (150g, 250g, 300g) washed once, the potassium iod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com