A kind of melelamycin enteric-coated capsule and its preparation method
A technology of mebelamycin and enteric-coated capsules, which is applied in the direction of medical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of lack of mebelamycin and low bioavailability , Dissolution and poor stability, etc., to achieve the effect of simple process, good enteric effect and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
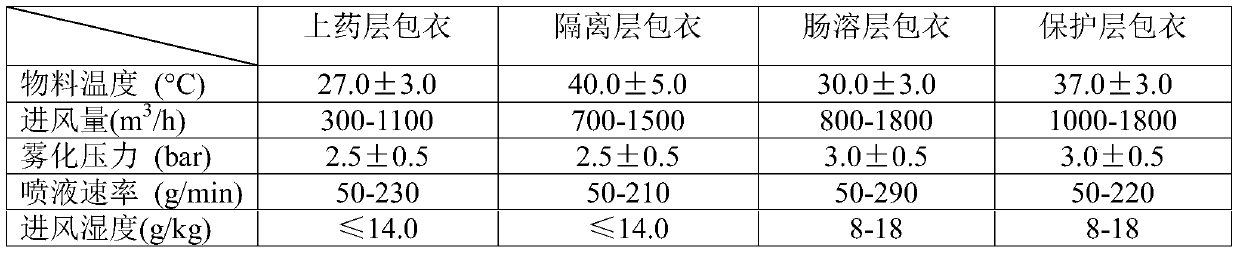
[0015] Weigh 40 parts of melemycin, 15 parts of copovidone, 6 parts of magnesium oxide, 800.5 parts of polysorbate, and 210 parts of isopropanol to prepare the coating solution for the coating layer; weigh 15 parts of copovidone, talc 32 parts of powder and 110 parts of purified water were used to prepare the coating solution for the isolation layer; 260 parts of acrylic resin, 6 parts of triethyl citrate, 3 parts of glyceryl monostearate, 800.5 parts of polysorbate, and 160 parts of purified water were weighed respectively. 3 parts of hydroxypropyl methylcellulose, 3 parts of talcum powder, and 60 parts of purified water were weighed to prepare the coating solution of the protective layer;
[0016] Add the blank pellet core into the GPCG30 multifunctional fluidized bed, and apply the above-mentioned coating solutions to the drug layer coating, isolation layer coating, enteric coating layer coating, and protective layer coating respectively. After sieving, Just dry.
[0017] ...
Embodiment 2
[0019] Weigh 45 parts of mebamycin, 16 parts of copovidone, 8 parts of magnesium oxide, 803 parts of polysorbate, and 220 parts of isopropanol to prepare the coating solution for the coating layer; weigh 16 parts of copovidone, talc 40 parts of powder and 120 parts of purified water were used to prepare the coating solution for the isolation layer; 270 parts of acrylic resin, 8 parts of triethyl citrate, 5 parts of glyceryl monostearate, 802 parts of polysorbate, and 170 parts of purified water were weighed respectively. 4 parts of hydroxypropyl methylcellulose, 5 parts of talcum powder, and 70 parts of purified water were weighed to prepare a coating solution for the protective layer; the blank ball core was added to the GPCG30 multifunctional fluidized bed Inside, each of the above-prepared coating solutions is respectively coated with a drug layer, an isolation layer, an enteric layer, and a protective layer, and then dried after sieving.
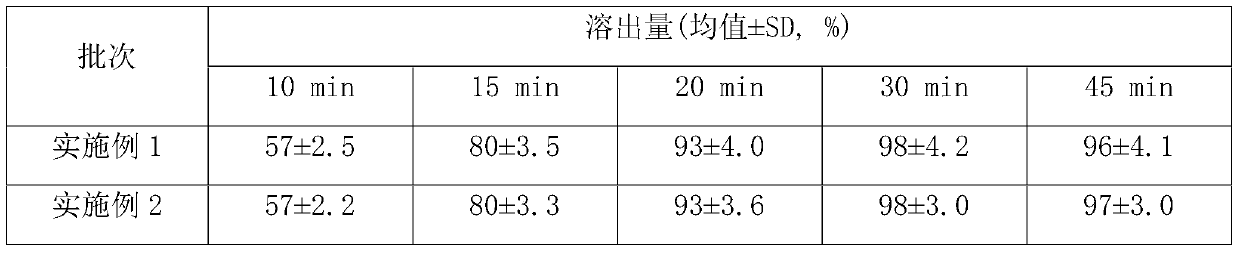
[0020] Twelve capsules were rando...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com