Cerium phosphate-based catalyst for zero-valent mercury oxidation, preparation method and application
A cerium phosphate-based, catalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, separation methods, etc., can solve the problems of large influence of zero-valent mercury oxidation activity, poor mercury oxidation effect, etc., to get rid of dependence. performance, easy operation, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
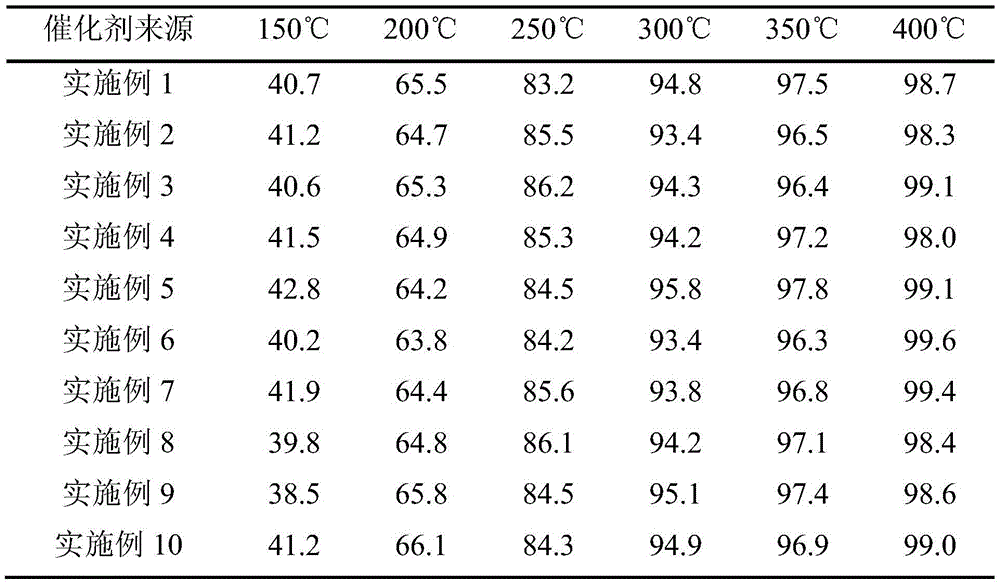
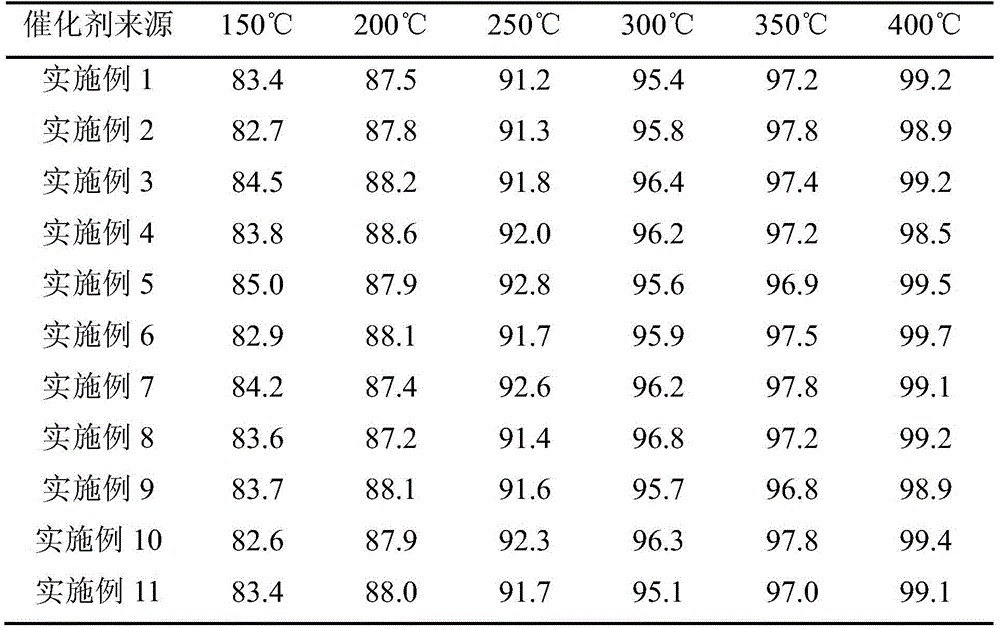
Examples
Embodiment 1
[0037] Catalyst preparation:
[0038] (1) Mix cerium nitrate hexahydrate and phosphoric acid at a molar ratio of 1:1, add a certain amount of urea (the molar ratio to cerium nitrate is 2 to 3), react at 80°C for 2 hours, cool the resulting mixture, let it stand, After washing, a white precipitate of cerium phosphate was obtained.
[0039] (2) Dissolve 0.6 mmol of cobalt nitrate in 200 ml of deionized water, add 0.02 mol of cerium phosphate obtained in step (1), and stir evenly. (the same below)
[0040] (3) After the mixture obtained in step (2) is dried, it is calcined at 300° C. for 8 hours in an air atmosphere to obtain a catalyst.
Embodiment 2
[0042] Catalyst preparation:
[0043] (1) Mix cerium nitrate hexahydrate and phosphoric acid at a molar ratio of 1:1, add a certain amount of urea (the molar ratio to cerium nitrate is 2 to 3), react at 80°C for 2 hours, cool the resulting mixture, let it stand, After washing, a white precipitate of cerium phosphate was obtained.
[0044] (2) Dissolve 0.6 mmol of manganese nitrate in 200 ml of deionized water, add 0.02 mol of cerium phosphate obtained in step (1), and stir evenly.
[0045] (3) After the mixture obtained in step (2) is dried, it is calcined at 300° C. for 8 hours in an air atmosphere to obtain a catalyst.
Embodiment 3
[0047] Catalyst preparation:
[0048] (1) Mix cerium nitrate hexahydrate and phosphoric acid at a molar ratio of 1:1, add a certain amount of urea (the molar ratio to cerium nitrate is 2 to 3), react at 90°C for 2 hours, cool the resulting mixture, let it stand, After washing, a white precipitate of cerium phosphate was obtained.
[0049] (2) Dissolve 0.6 mmol of copper nitrate in 200 ml of deionized water, add 0.02 mol of cerium phosphate obtained in step (1), and stir evenly.
[0050] (3) After the mixture obtained in step (2) is dried, it is calcined at 300° C. for 8 hours in an air atmosphere to obtain a catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com