Method for preparing load type nanogold catalyst
A nano-gold and catalyst technology, which is applied in the field of preparation of supported nano-gold catalysts, can solve problems such as single gold anchor point properties, catalyst catalytic activity, catalyst stability constraints, etc., and achieve good catalytic activity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation method of supported nanometer gold catalyst comprises the steps:
[0025] step one
[0026] at 60 o Under the condition of C, take 19% polyoxypropylene polyoxyethylene copolymer solution and dissolve it in deionized water to form cloudy solution A;
[0027] step two
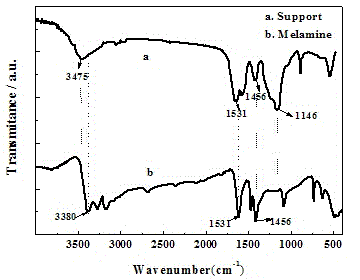
[0028] Prepare 15% formaldehyde into a 37 wt.% formaldehyde aqueous solution, then mix it with deionized water to obtain a cloudy solution B, then adjust the pH value of solution B to 8.7 with sodium hydroxide aqueous solution, and then add 11% Melamine and 7% thiourea, at 60 o C was stirred for 2 hours to obtain solution C;
[0029] step three
[0030] Pour solution A into solution C and stir for 2.5 hours to obtain solution D. During the stirring of solution D, dissolve 40% water glass in deionized water and stir at room temperature for about 30 minutes to obtain solution E. In solution D Quickly pour 8% glacial acetic acid, under vigorous stirring, immediately pour solution E into ...
Embodiment 2
[0034] The preparation method of supported nanometer gold catalyst is characterized in that comprising the steps:
[0035] step one
[0036] at 70 o Under the condition of C, take 23% polyoxypropylene polyoxyethylene copolymer solution and dissolve it in deionized water to form cloudy solution A;
[0037] step two
[0038] Prepare 17% formaldehyde into a formaldehyde aqueous solution with a concentration of 37 wt.%, then mix it with deionized water to obtain a cloudy solution B, then adjust the pH value of solution B to 8.5-9.0 with aqueous sodium hydroxide solution, and then add 9 % of melamine and 6% of thiourea, at 50 o C was stirred for 3 hours to obtain solution C;
[0039] step three
[0040] Pour solution A into solution C and stir for 3 hours to obtain solution D. During the stirring of solution D, dissolve 38% water glass in deionized water and stir at room temperature for about 35 minutes to obtain solution E. In solution D Quickly pour 7% glacial acetic acid, ...
Embodiment 3
[0044] The preparation method of supported nanometer gold catalyst is characterized in that comprising the steps:
[0045] step one
[0046] at 50 o Under the condition of C, take 15% polyoxypropylene polyoxyethylene copolymer solution and dissolve it in deionized water to form cloudy solution A;
[0047] step two
[0048] Prepare 13% formaldehyde into a 37 wt.% formaldehyde aqueous solution, mix it with deionized water to obtain a cloudy solution B, adjust the pH value of solution B to 8.5-9.0 with sodium hydroxide aqueous solution, and then add 13 % of melamine and 8% of thiourea, at 70 o C was stirred for 1 hour to obtain solution C;
[0049] step three
[0050] Pour solution A into solution C and stir for 2 hours to obtain solution D. During the stirring of solution D, dissolve 42% water glass in deionized water and stir at room temperature for about 25 minutes to obtain solution E. In solution D Quickly pour 9% glacial acetic acid, under vigorous stirring, immediately...
PUM
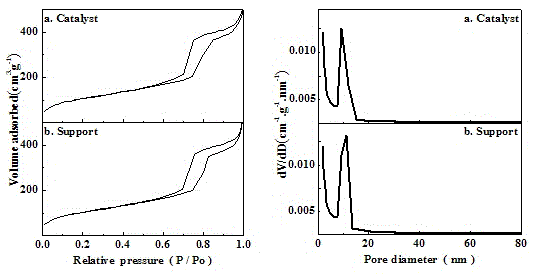
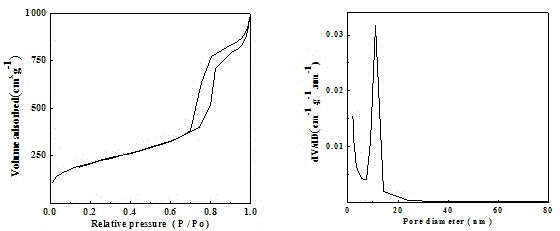
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com