An annulene derivative containing an exocyclic double bond and a preparation method thereof
A technology of derivatives and annulene, applied in the field of annulene derivatives and their preparation, can solve problems such as non-existent preparation methods and complex structures, and achieve broad application prospects and complex and diverse structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
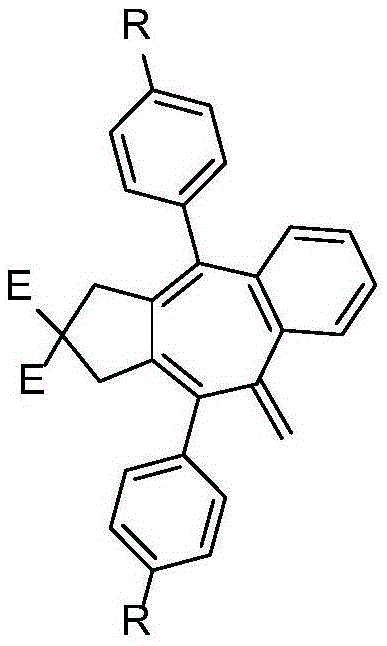
[0029] An annulene derivative containing an exocyclic double bond, the structural formula of the annulene derivative containing an exocyclic double bond is:
[0030]
[0031] Among them, E 1 =E 2 =CO 2 R;
[0032] R is straight-chain alkyl, branched-chain alkyl;
[0033] R 1 , R 2 It is halogen, straight chain alkyl, branched chain alkyl, ester group, alkoxy group and their corresponding derivatives.
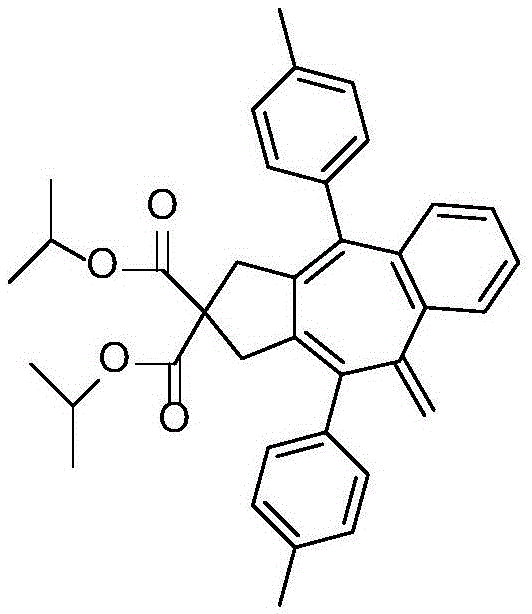
[0034] Preferably, the structural formula of an annulene derivative containing an exocyclic double bond is:
[0035]
[0036] The R is isopropyl, R 1 , R 2 For methyl.
[0037] The annulene derivative containing an exocyclic double bond is a multi-substituted annulene derivative containing an exocyclic double bond.
[0038] A method for preparing an annulene derivative containing an exocyclic double bond, comprising the following steps:
[0039] a. Precursor synthesis;
[0040] (1) With sodium hydride as a catalyst, diisopropyl malonate and propargyl bromide we...
Embodiment 2
[0047] An annulene derivative of an exocyclic double bond, its structural formula is:
[0048]
[0049] where E 1 =E 2 =CO 2 R, R is straight-chain alkyl, branched-chain alkyl, saturated hydrocarbon, unsaturated hydrocarbon or aromatic hydrocarbon group; R 1 , R 2 It is halogen, straight chain alkyl, branched chain alkyl, ester group, alkoxy group and their corresponding derivatives.
[0050] A preparation method of an annulene derivative containing an exocyclic double bond, comprising the following steps:
[0051] (1) Using sodium hydride as a catalyst, diisopropyl malonate and propargyl bromide were added to anhydrous acetonitrile in an ice-water bath, stirred and reacted, and a white solid product, namely compound 1, was obtained after purification and separation;
[0052] The molar ratio of diethyl malonate to propargyl bromide is 1:2.2-3.2;
[0053] The purification and separation is to wash the product with water, extract with ethyl acetate, spin dry under reduc...
Embodiment 3
[0059] An annulene derivative containing an exocyclic double bond, the structural formula is:
[0060]
[0061] A preparation method of an annulene derivative containing an exocyclic double bond, the preparation method comprising the following steps:
[0062] a. Precursor synthesis;
[0063] b. Synthesis of the target product;
[0064] c. Purification.
[0065] Wherein, a, precursor synthesis, comprises the following steps:
[0066] (1) Using sodium hydride as a catalyst, add 200mmol diisopropyl malonate and 440mmol propargyl bromide to an ice-water bath in anhydrous acetonitrile, stir and react for 8 hours, wash the product with water, extract with ethyl acetate, and depressurize Spin to dry, column chromatography (volume ratio ethyl acetate:petroleum ether=1:100) to obtain a white solid product, namely compound 1;
[0067]
[0068] (2) Under anhydrous and oxygen-free conditions, 20mmol of compound 1, 48mmol of p-methyl iodobenzene, 842mg (1.2mmol) of PdCl 2 (PPh) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com