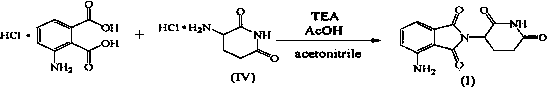
Method for preparing pomalidomide by one-pot process
A pomalidomide and compound technology, applied in the field of pomalidomide preparation, can solve the problems of compound III instability, large environmental pollution, harsh reaction conditions, etc., reduce material transfer and unit operations, reduce production costs, The effect of reagent safety and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0034] Example 1: One-pot preparation of pomalidomide
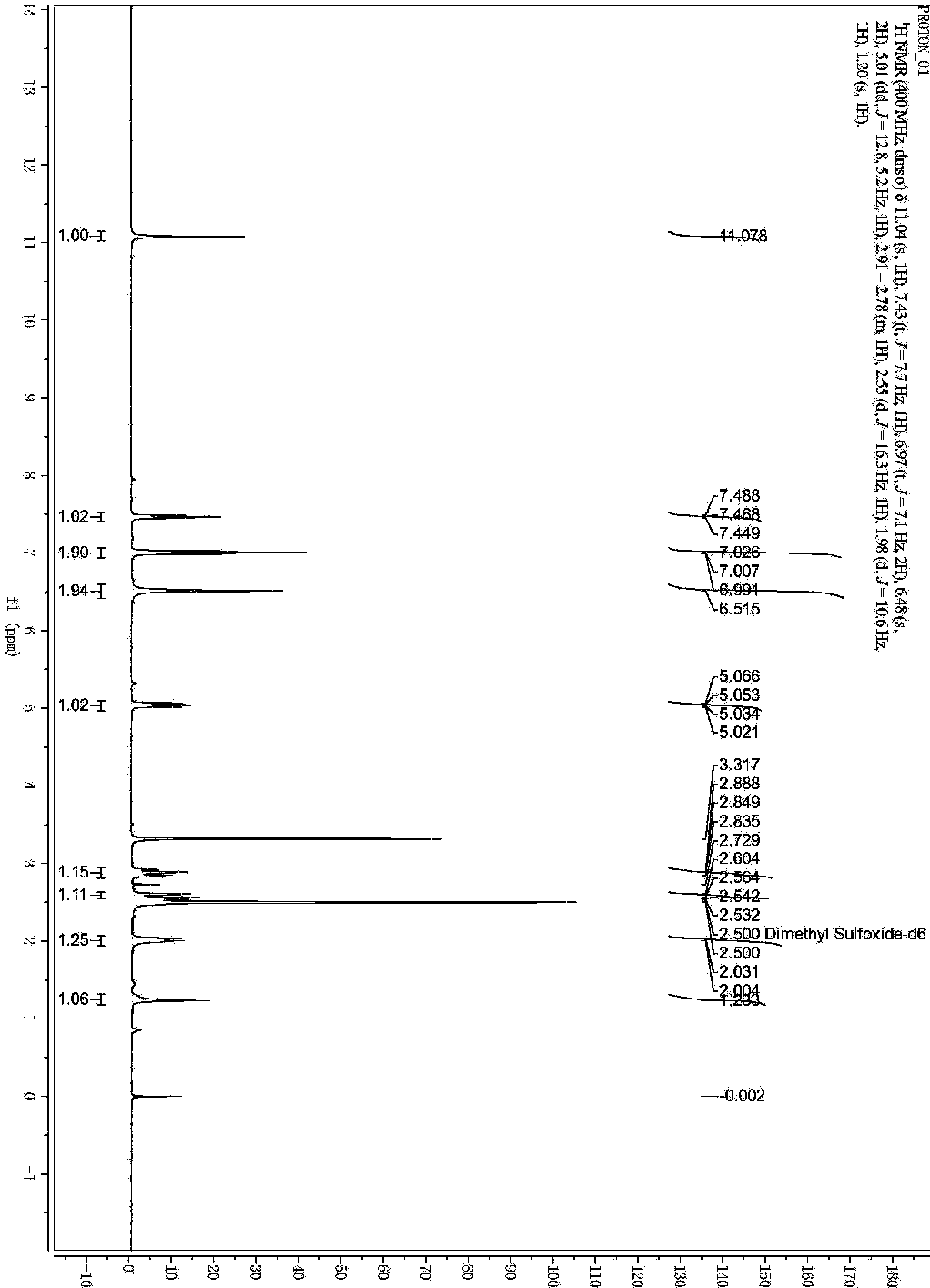
[0035] Add 211g of 3-nitrophthalic acid into the reaction flask, add 100ml of acetic anhydride and 650ml of acetic acid, stir and mix, and heat to reflux at 120°C for 16h. Add 165g of 3-aminopiperidine-2,6-dione hydrochloride, 90g of sodium acetate, and 2500ml of acetic acid, heat to reflux at 120°C for 8 hours, then cool to 75±2°C. Add 240g of iron powder in batches, control the reaction temperature not to exceed 105°C, stir the reaction for 5 hours, and cool to room temperature. After filtering, the filter cake was washed with water, and then beaten and washed with sodium bicarbonate solution, and the crude product was obtained after filtration. The crude product was dissolved by heating with 2400ml dimethyl sulfoxide and filtered. Water was added dropwise to the filtrate, the precipitated solid was collected by filtration, and air-dried at 60° C. for 4 h. 216.7 g of yellow solid was obtained, yield: 79.3%, purity: 9...
example 2
[0038] Example 2: One-pot preparation of pomalidomide
[0039] Add 21.1g of 3-nitrophthalic acid into the reaction flask, add 10ml of acetic anhydride and 65ml of acetic acid, stir and mix, and heat to reflux at 120°C for 15h. Add 16.5g of 3-aminopiperidine-2,6-dione hydrochloride, 10g of sodium acetate, and 250ml of acetic acid, heat to reflux at 120°C for 6 hours, then cool to 75±2°C. Add 24g of iron powder in batches, control the reaction temperature not to exceed 105°C, stir the reaction for 5 hours, and cool to room temperature. Filtrate, wash the filter cake with water, and then beat and wash with sodium bicarbonate solution. After filtration, the crude product is obtained. The crude product is heated and dissolved in 240ml of dimethyl sulfoxide, filtered, and water is added dropwise to the filtrate. 20.8 g of yellow solid was obtained, yield: 76.2%, purity: 99.96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com