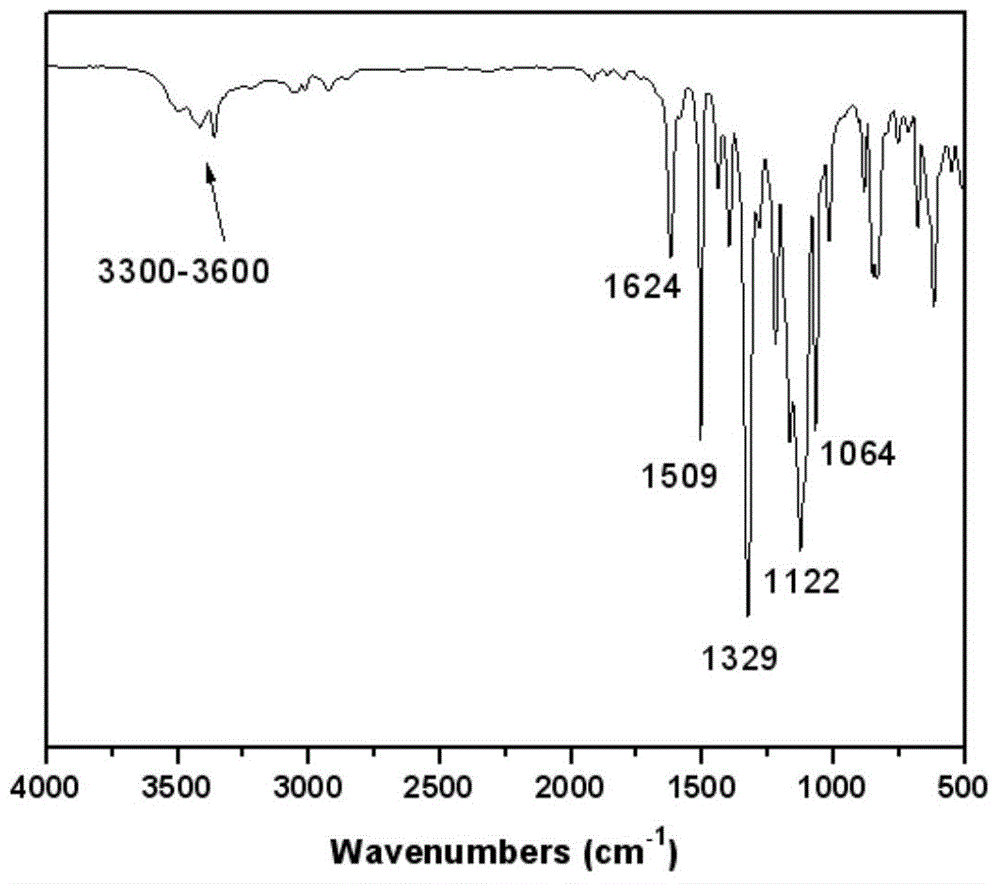
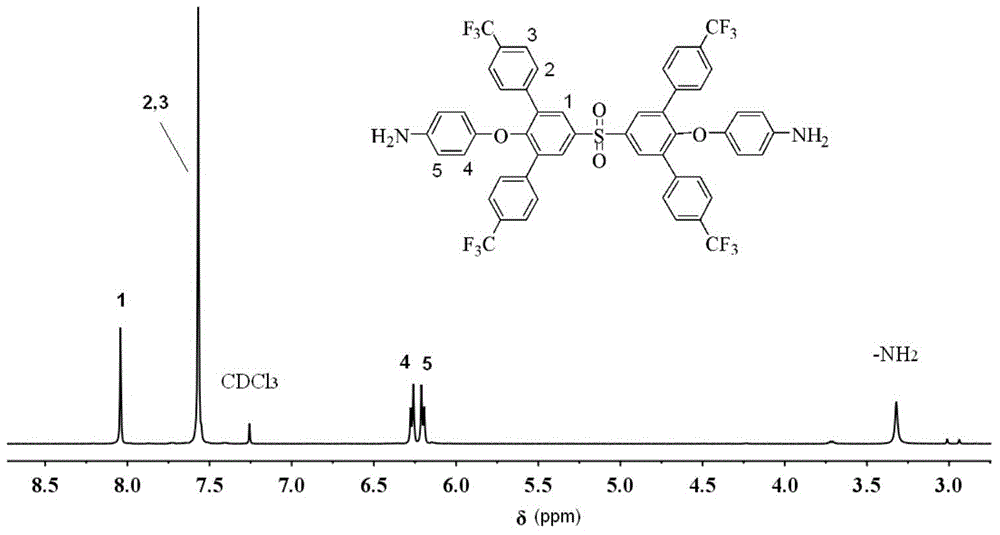
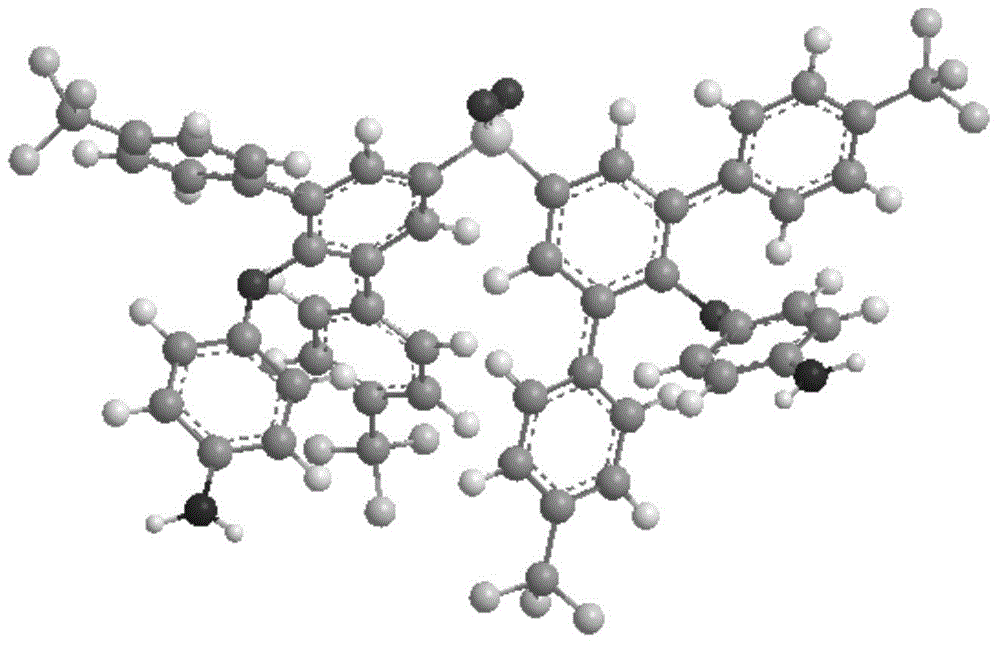
Aromatic diamine monomer simultaneously containing four lateral substituents and having twisted non-coplanar structure and preparation method thereof
An aromatic diamine and non-coplanar technology, which is applied in the field of aromatic diamine monomers and their preparation, can solve difficult problems such as easy purification and separation, simple synthesis route, and improved gas separation performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Add 28.492g (0.05mol) of 2,2'-bis(3,5-dibromo-4-fluorophenyl) to a 1000ml three-necked flask equipped with mechanical stirring, condensing device and nitrogen protection Sulfone and 37.986g (0.20mol) of 4-trifluoromethylphenylboronic acid, 2.311g (0.002mol) of tetrakis (triphenylphosphine) palladium, 42.396g (0.40mol) of sodium carbonate, 170g of water, 85ml of toluene and 85ml of ethylene glycol dimethyl ether, stirred at room temperature (25°C) for 10 minutes, then heated to reflux, reacted for 8 hours, evaporated the organic solvent, and filtered the crude product, washed with water and dried, and used N,N- Further recrystallization of dimethylformamide gave the intermediate bisfluoro compound 2,2'-bis[3,5-bis(4-trifluoromethylphenyl)-4-fluorophenyl]sulfone as a white powder, producing The rate was 81% (calculated based on the conversion rate of 2,2'-bis(3,5-dibromo-4-fluorophenyl)sulfone).
[0024] The melting point of the 2,2'-bis[3,5-bis(4-trifluoromethylphen...
Embodiment 2
[0029] (1) Add 28.492g (0.05mol) of 2,2'-bis(3,5-dibromo-4-fluorophenyl) to a 1000ml three-necked flask equipped with mechanical stirring, condensing device and nitrogen protection Sulfone and 41.785g (0.22mol) of 4-trifluoromethylphenylboronic acid, 3.466g (0.003mol) of tetrakis (triphenylphosphine) palladium, 52.995g (0.50mol) of sodium carbonate, 265g of water, 158ml of toluene and 158ml of ethylene glycol dimethyl ether, stirred at room temperature (25°C) for 10 minutes, then heated to reflux, reacted for 16 hours, evaporated the organic solvent, and filtered the crude product, washed with water, dried, and used N,N- Further recrystallization of dimethylformamide gave the intermediate bisfluoro compound 2,2'-bis[3,5-bis(4-trifluoromethylphenyl)-4-fluorophenyl]sulfone as a white powder, producing The rate was 82% (calculated based on the conversion rate of 2,2'-bis(3,5-dibromo-4-fluorophenyl)sulfone).
[0030](2) In a 250ml three-neck flask equipped with mechanical stirrin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com