Preparation method of high-purity 4,5-dicyano-2-trifluoromethylimidazole and salts thereof
A technology of trifluoromethyl imidazole salt and trifluoromethyl imidazole is applied in the field of preparation of high-purity 4,5-dicyano-2-trifluoromethyl imidazole and its salt, and can solve the problem of volatile, measurement deviation , inconvenient use and other problems, to achieve the effect of improving yield, mild process conditions and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
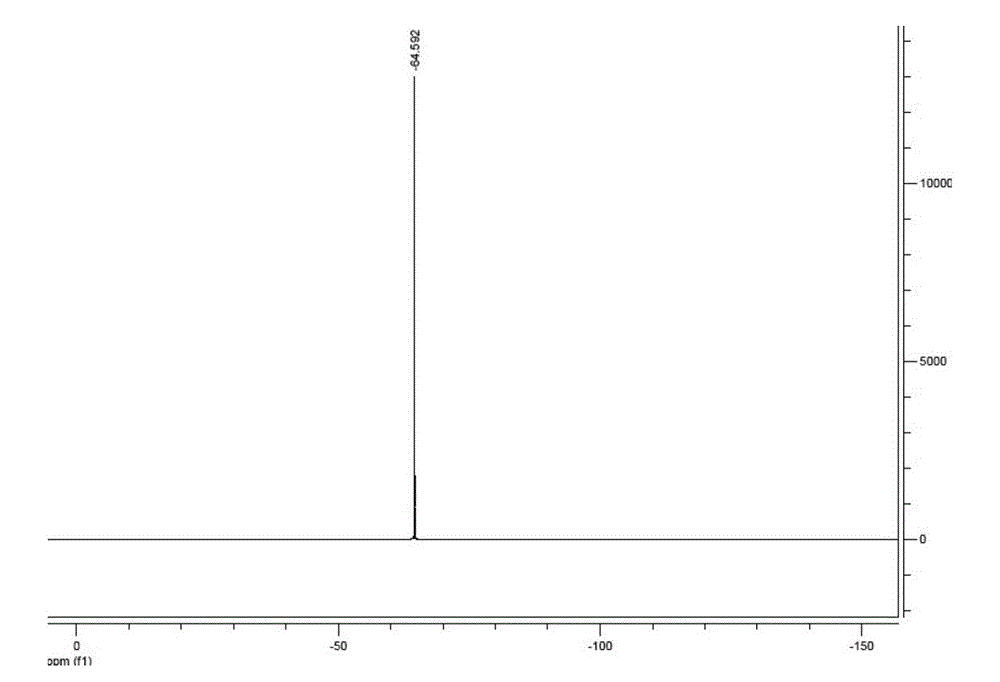
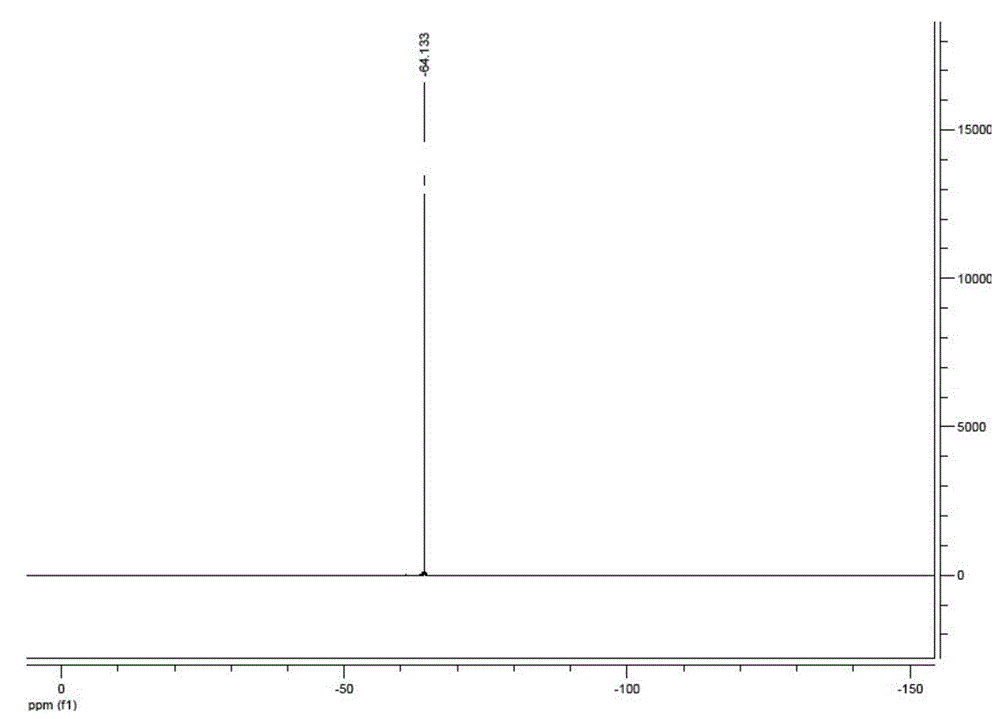
[0043] In a 1000 ml three-neck flask equipped with an electric stirrer, a reflux condenser, and a thermometer, 43.24 g (0.4 mol) of diaminomaleonitrile, 600 mL of 1,4-dioxane, and 50.17 g of trifluoroacetic acid ( 0.44mol), P 2 o 5 28.39g (0.2mol), heated to 80°C for 3h. After the reaction was completed, the solvent and most of the remaining trifluoroacetic acid were removed by evaporation, 600 ml of water was added to the system, and 20 g of activated carbon was added to remove the color. The filtrate was concentrated to 200 mL, crystallized by cooling, filtered to obtain crystals and washed with 100 mL of ice water, the obtained crystals were recrystallized with 200 mL of water, filtered to obtain crystals and washed with 100 mL of ice water. The resulting filtrate and washings were used in the next purification. The crystals were dried at 60°C for 6 hours under reduced pressure and then used 19 F NMR (solvent is acetonitrile) detection, no obvious impurity peak ( figure ...
Embodiment 2
[0045] In a 1000 ml three-neck flask equipped with an electric stirrer, a reflux condenser, and a thermometer, 43.24 g (0.4 mol) of diaminomaleonitrile, 600 mL of 1,4-dioxane, and 91.22 g of trifluoroacetic acid ( 0.8mol), P 2 o 5 56.78g (0.4mol), heated to 120°C for 1h. After the reaction was completed, the solvent and most of the remaining trifluoroacetic acid were removed by evaporation, and 600 ml of the filtrate and washing liquid from the previous experiment were added to the system, and 25 g of activated carbon was added to remove the color. The filtrate was concentrated to 200 mL, crystallized by cooling, filtered to obtain crystals and washed with 100 mL of ice water, the obtained crystals were recrystallized with 200 mL of water, filtered to obtain crystals and washed with 100 mL of ice water. The resulting filtrate and washings were used in the next purification. The crystals do not need to be dried, and LiOH aqueous solution is added thereto until the pH is 7. ...
Embodiment 3
[0047] In a 1000 ml three-neck flask equipped with an electric stirrer, a reflux condenser, and a thermometer, 43.24 g (0.4 mol) of diaminomaleonitrile, 500 mL of 1,4-dioxane, and 228.05 g of trifluoroacetic acid ( 2mol), P 2 o 5 85.17g (0.6mol), heated to 100°C for 2h. After the reaction was completed, the solvent and most of the remaining trifluoroacetic acid were removed by evaporation, and 600 ml of the filtrate and washing liquid from the previous experiment were added to the system, and 25 g of activated carbon was added to remove the color. The filtrate was concentrated to 200 mL, crystallized by cooling, filtered to obtain crystals and washed with 100 mL of ice water, the obtained crystals were recrystallized with 200 mL of water, filtered to obtain crystals and washed with 100 mL of ice water. The resulting filtrate and washings were used in the next purification. The crystal does not need to be dried, Na is added to it 2 CO 3 Aqueous solution, stop when the pH i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com