Method for preparing high-purity EGCG from hydrogen-bonded macroporous resin
A macroporous resin and high-purity technology, applied in the field of preparation of high-purity EGCG, can solve the problems that toxic chemical reagents cannot be guaranteed and are not suitable for large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0023] Detailed ways: The present invention is described in detail through the following examples, but the technical solution of the present invention is not limited to the following examples.
[0024] Among the present invention, the HPLC assay method is as follows:
[0025] Type of instrument used: high performance liquid chromatography Elution method: gradient elution;
[0026] Detector: UV detector Instrument model: Agilent 1200;
[0027] Detection wavelength: 278nm Injection volume: 20μL Flow rate: 1mL / min;
[0028] Column temperature: 30°C Column type: Diamonsil C18 column (150mm×4.6mm, 5μm);
[0029] Integral method: area normalization method.
[0030] Liquid chromatography gradient elution process:
[0031] time (min) Methanol (A) 0.8% glacial acetic acid in water (B) 0 10 90 1 15 85 11 18 82 21 21 79 51 70 30
Embodiment 1
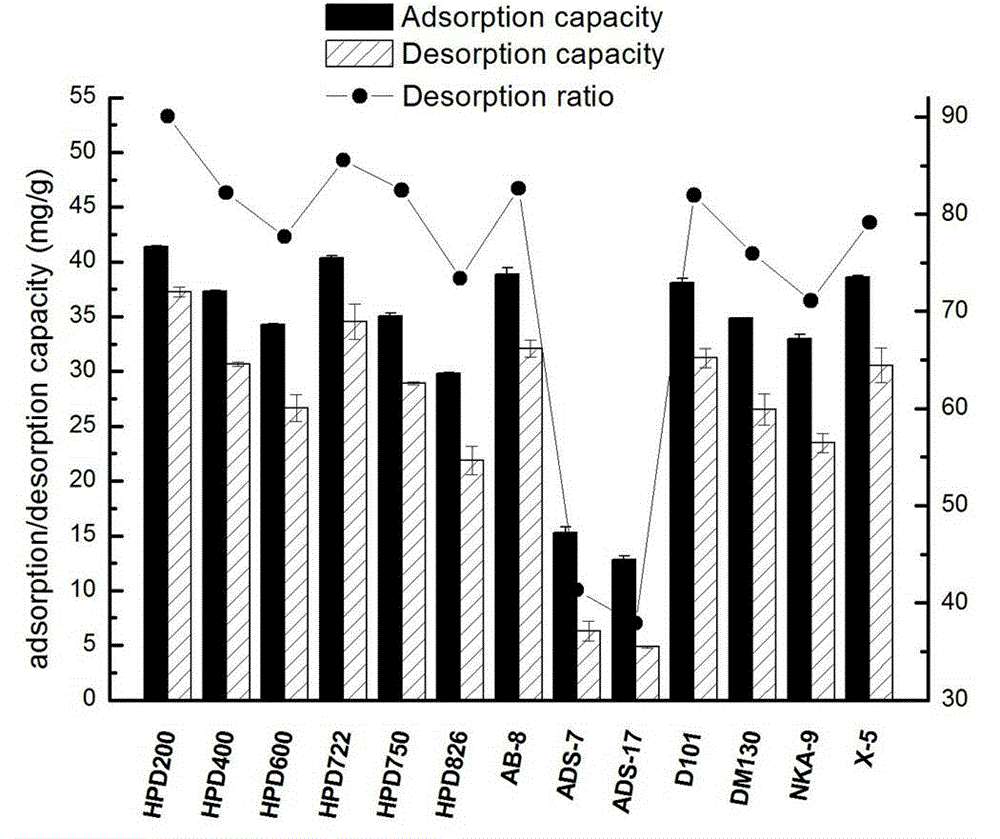
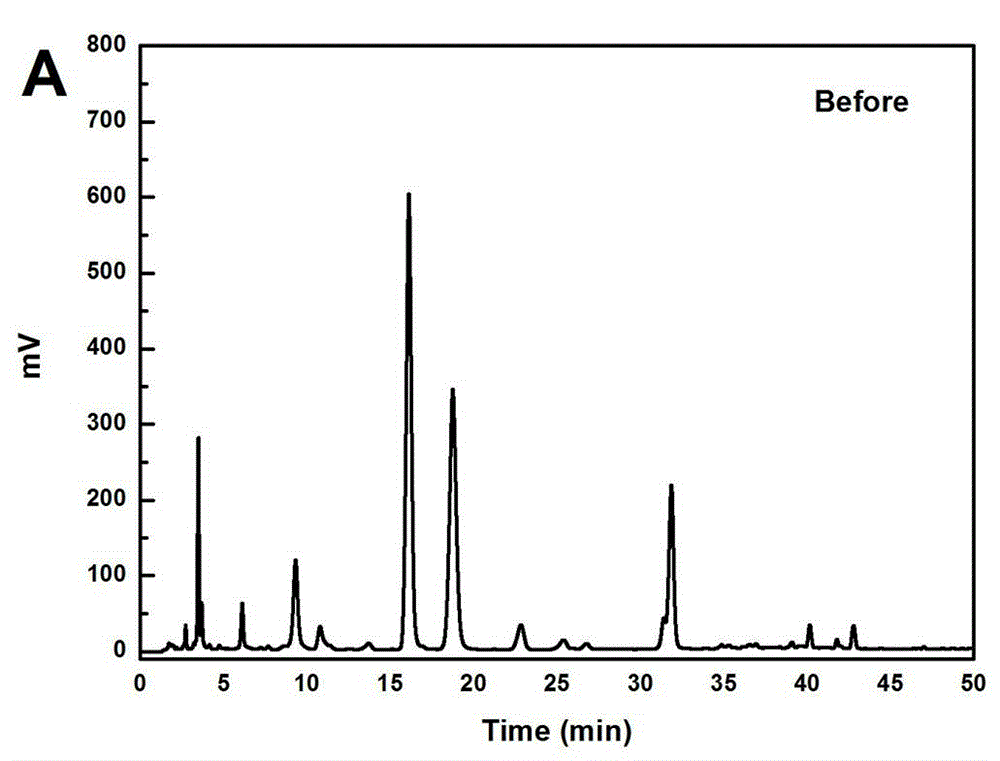
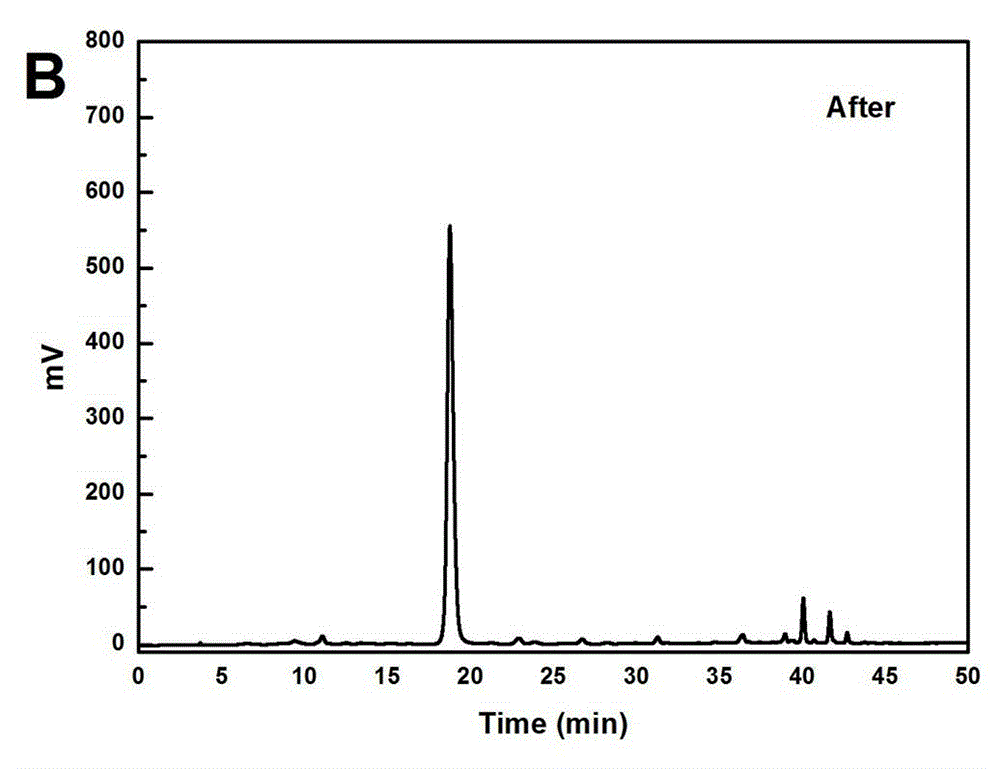
[0033] Take 500g of green tea, add 10L of water, heat to 60°C for extraction, extract for 1 hour, extract 3 times, add the extract to the treated HPD-826 hydrogen bond type macroporous adsorption resin column for adsorption, wash with 5 BV of water Removed, discarded, and then eluted with 5 BV 20% ethanol water, recovered the solvent, discarded the residue, then eluted with 10 BV 30% ethanol water, collected 30% eluate, concentrated under reduced pressure, and freeze-dried to obtain EGCG crude product 10.69g, such as image 3 shown; the crude product was recrystallized with ethanol:water:acetic acid (9:1:1) to obtain 6.94g of EGCG, and the content of EGCG was 98.60% by HPLC analysis, as attached Figure 4 shown.
Embodiment 2
[0035] Take 500g of green tea, add 20L of water, heat to 70°C for extraction, extract for 2 hours, extract 3 times, add the extract to the treated HPD-200 hydrogen bond type macroporous adsorption resin column for adsorption, and elute with 5BV of water , discarded, and then eluted with 5 BV of 20% ethanol water, the solvent was recovered, the residue was discarded, and then eluted with 10 BV of 30% ethanol water, the 30% eluate was collected, concentrated under reduced pressure, and freeze-dried to obtain EGCG crude product 15.85g, the crude product was recrystallized with ethanol:water:acetic acid (8:2:1) to obtain 9.12g of EGCG, the content of EGCG was 98.32% by HPLC analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com