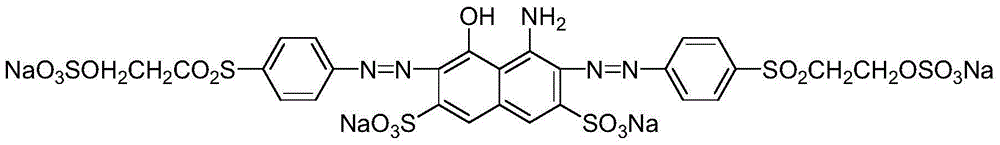
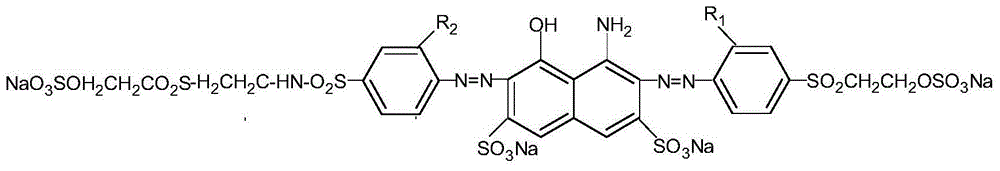
Compound capable of being used as reactive navy blue dye and preparation method of compound
A compound and reactive technology, applied in the field of compounds that can be used as reactive navy blue dyes and their preparation, can solve the problems of rubbing fastness only 1-2 grades, which cannot be achieved, and achieve bright color and low directness. , the effect of less isomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Nitrogen induction reaction
[0041] Add 28.1 parts by weight of 4-(β-hydroxyethylsulfone sulfate) aniline to 100 parts of ice water, add 0.1 part of JS-C wetting agent at the same time, beat at about 0°C for 2 hours, then add 35 parts of hydrochloric acid, Add ice to control the temperature at 0-5°C. Slowly add 6.9 parts of sodium nitrite (made into a 30% solution) in a trickle under the liquid surface, and control the reaction solution in the process of adding sodium nitrite solution to be blue to Congo red test paper, and to be slightly blue to starch KI test paper. After the sodium solution is added, keep a slight excess, and react at a temperature of 0-5°C for 1 hour, and use a small amount of sulfamic acid to eliminate the slight excess of nitrous acid before acid coupling.
[0042] 2. Acid couple reaction
[0043] Add 33.2 parts of H acid powder industrial product to the above diazonium salt in 4 times, and react at 10-15°C until the diazonium salt disappear...
Embodiment 2
[0051] 1. Nitrogen induction reaction
[0052] Add 28.16 parts by weight of 4-(β-hydroxyethylsulfone sulfate) aniline to 100 parts of ice water, add 0.1 part of JS-C wetting agent at the same time, beat at about 0°C for 2 hours, then add 35.9 parts of hydrochloric acid, Add ice to control the temperature at 0-5°C. Slowly add 7.0 parts of sodium nitrite (made into a 30% solution) in a trickle under the liquid surface. During the addition of sodium nitrite solution, the reaction solution is controlled to be blue to Congo red test paper, and slightly blue to starch KI test paper. After the sodium solution is added, keep a slight excess, and react at a temperature of 0-5°C for 1 hour, and use a small amount of sulfamic acid to eliminate the slight excess of nitrous acid before acid coupling.
[0053] 2. Acid couple reaction
[0054] Add 33.9 parts of H acid powder industrial product to the above diazonium salt in 5 times, and react at 10-15°C until the diazonium salt disappears....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com