Pharmaceutical composition for treating inflammatory demyelinating diseases of central nervous system and application of combined administration thereof
A demyelinating disease, central nervous system technology, applied in nervous system diseases, drug combinations, allergic diseases, etc., can solve the problems of lack of specific drug treatment and incomplete elucidation of the pathogenesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
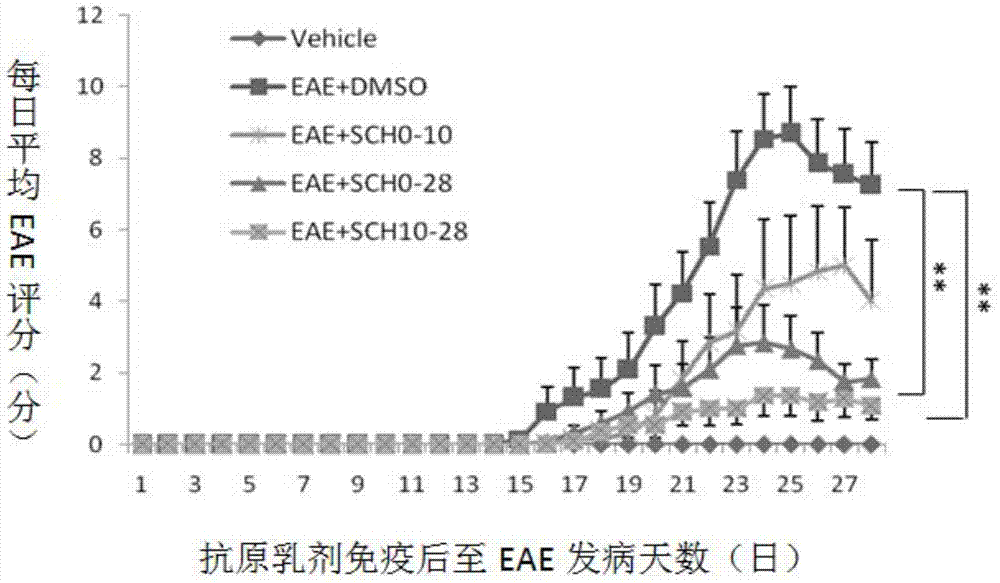
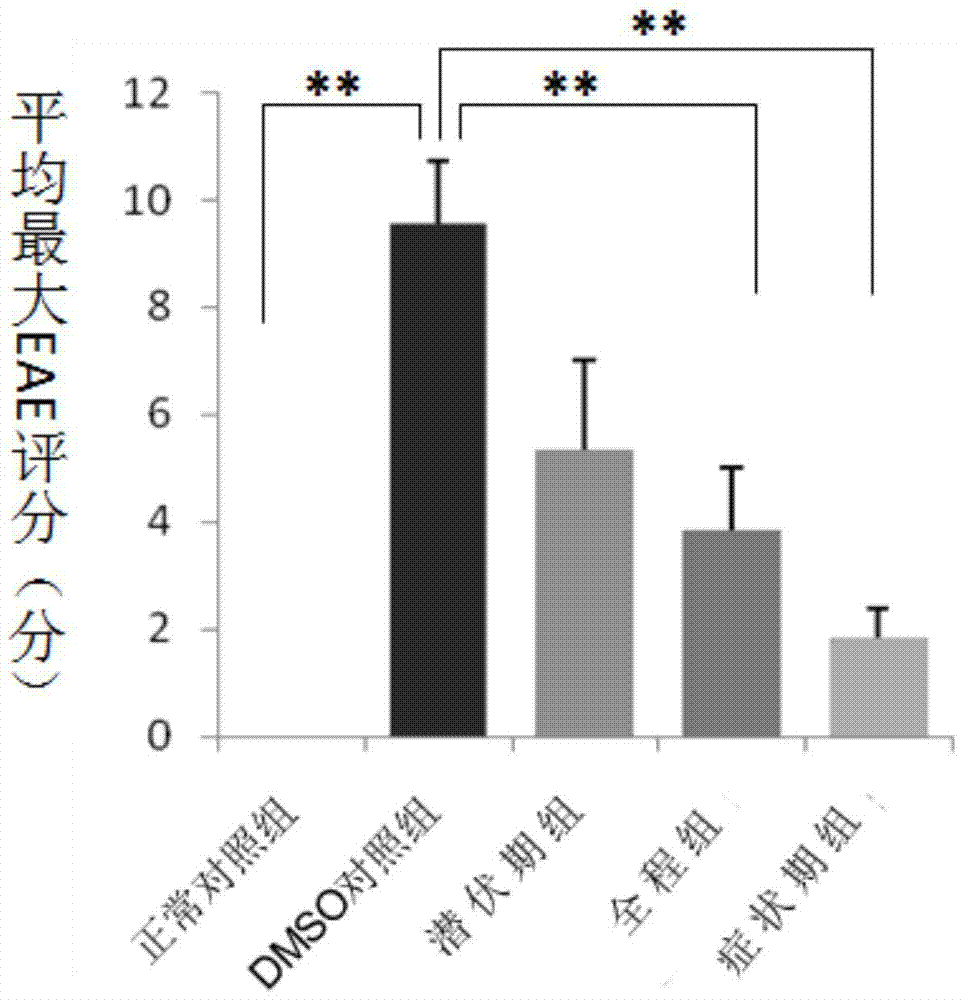
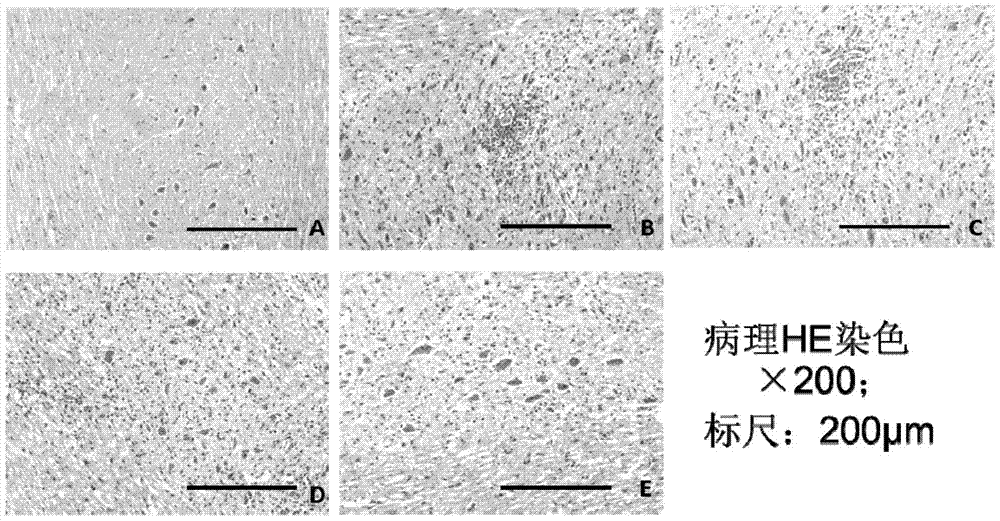
[0066] Example 1. Study on the Mechanism of SCH in Treating Central Nervous System Inflammatory Demyelinating Diseases
[0067] 1. Making of EAE model
[0068] Of 48 C57BL / 6 mice, 42 of them were randomly selected, and each mouse was anesthetized with 0.5% pentobarbital (0.27ml) intraperitoneally, and then intradermally injected with antigen at four points at the base of the tail, both sides of the abdomen, and the neck. Emulsion (mixed with myelin oligodendrocyte glycoprotein (MOG) and complete Freund's adjuvant), inject 0.05ml at each point, 0.2ml in total, and then intraperitoneally inject pertussis toxin immediately and 48 hours later. , PT) 500ng to induce an immune response to create a classic EAE model.
[0069] 2. Animal Grouping and Drug Intervention
[0070]Six mice that were not injected with MOG antigen emulsion were used as the normal control group; 42 EAE model mice that were injected with MOG antigen emulsion were randomly divided into DMSO control group, incu...
Embodiment 2
[0105] Example 2. Combined use of 2-BFI and SCH in the treatment of central nervous system inflammatory demyelinating diseases
[0106] 1. Making of EAE model
[0107] There were 64 C57BL / 6 mice, and each mouse was anesthetized with 0.5% pentobarbital 0.27ml intraperitoneally, and then injected MOG antigen emulsion intradermally at the base of the tail, both sides of the abdomen, and the neck (injection at each point) 0.05ml, 0.2ml in total). Immediately and 48 hours after the injection, 500 ng of pertussis toxin (PT) was injected intraperitoneally, respectively, to induce an immune response, and to create a classic EAE model.
[0108] 2. Animal Grouping and Drug Intervention
[0109] The EAE model mice injected with MOG antigen emulsion and immunized were randomly divided into 6 groups, and SCH was dissolved in 5% DMSO as a vehicle to make a 0.02% SCH solution for later use; 2-FBI was dissolved in 0.9% normal saline to make a concentration of 0.2 %2-BFI solution for later ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com