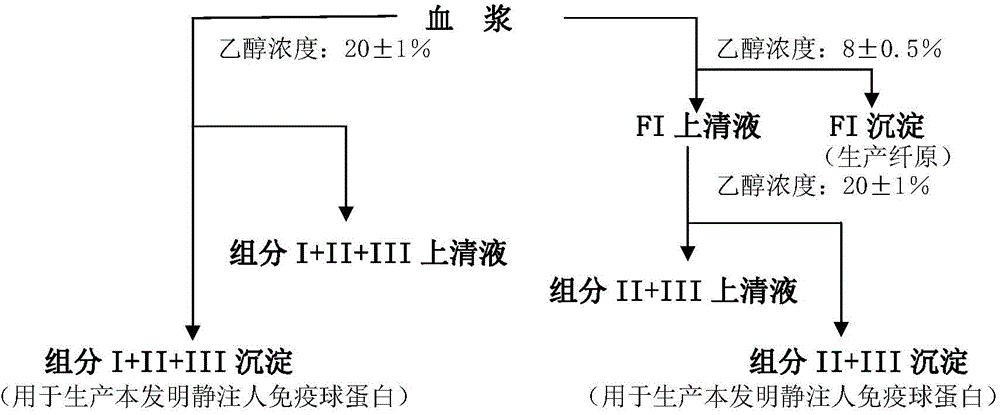
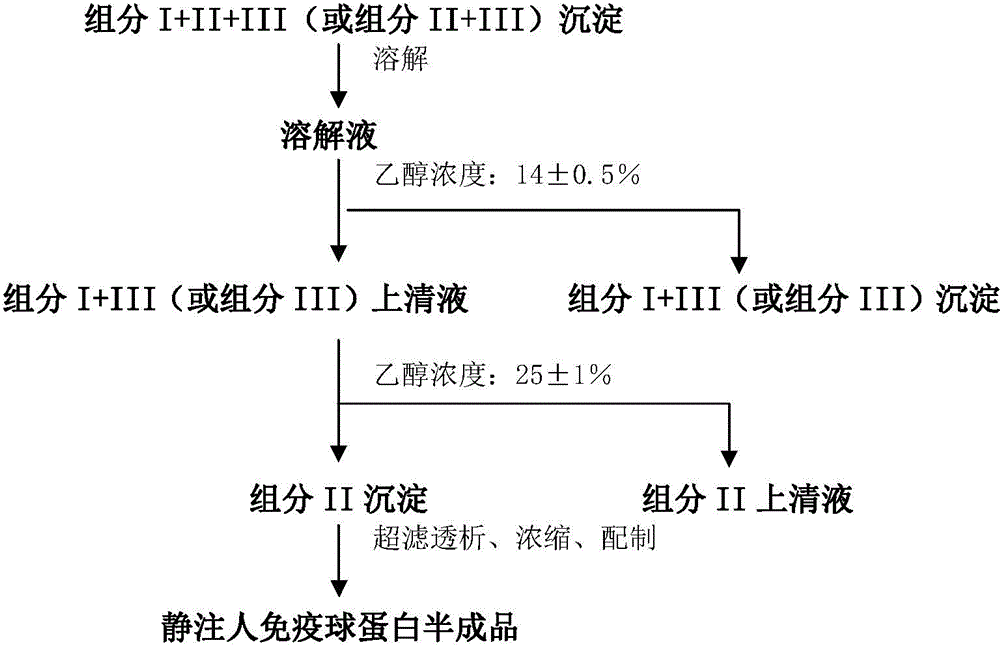
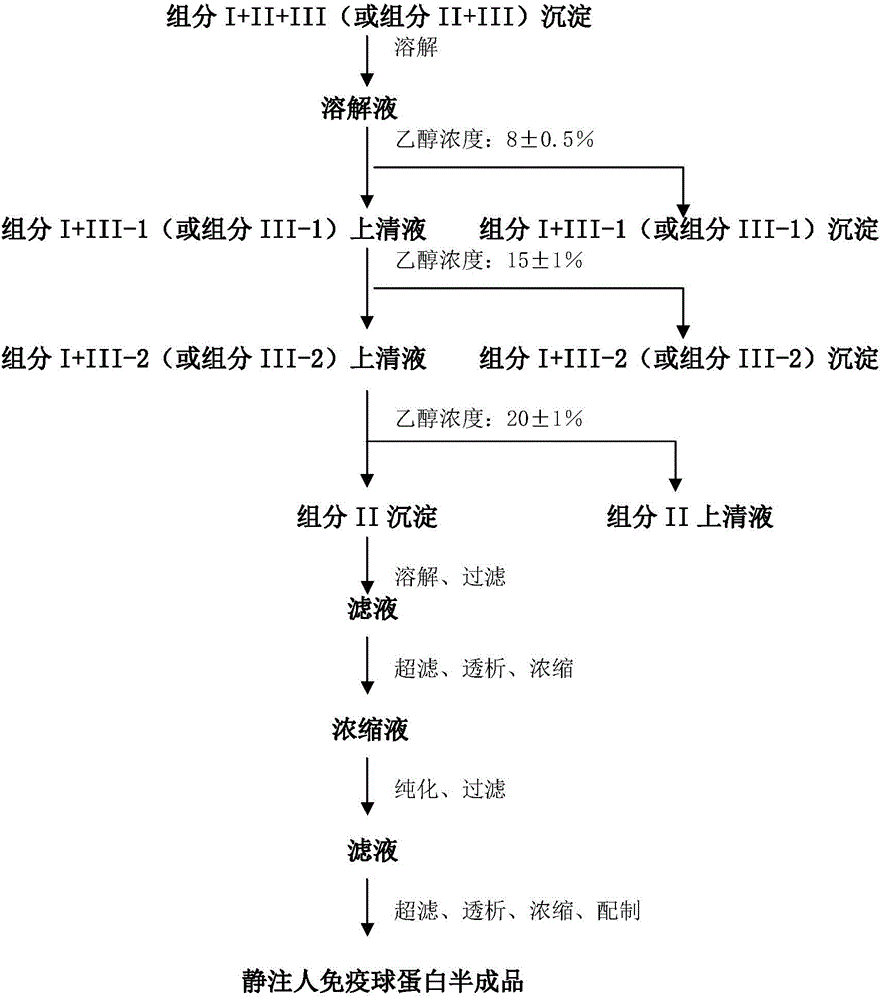
Method for preparing intravenous immunoglobulin
A technology of intravenous injection of human immunoglobulin, which is applied in the field of preparation of intravenous human immunoglobulin, can solve the problems of low purity, increased risk, and adverse reactions of intravenous injection of human immunoglobulin, so as to reduce the risk of medication and ensure medication safe effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Step 1: Dissolution of Component I+II+III Precipitate
[0089] 56.3 kg of components I+II+III were stirred with 394.54 kg of 0.7°C aqueous solution containing 0.54 kg of sodium dihydrogen phosphate (containing 0.54 kg of sodium dihydrogen phosphate and 394.0 kg of water for injection) for more than 6 hours until completely dissolved to obtain the composition Divide 450.8kg of I+II+III precipitation solution.
[0090] Step 2: Separation of Component I+III-1 Precipitation
[0091] Use 3.0kg of 1mol / L acetic acid solution to adjust the pH value of the above component I+II+III precipitation solution to 5.03, use -15°C refrigerant to cool down to -0.4°C, and then add -20.8°C 95% (v / v) ethanol Solution 28.2kg, and use 0.5mol / L sodium hydroxide solution to adjust the pH value to 5.12, use -15°C refrigerant to adjust the final suspension temperature to -2.6°C, stir at a stirring speed of 90rpm for 2 hours, add diatomaceous earth after the reaction is complete 1.9kg and 1.2kg ...
Embodiment 2
[0111] Step 1: Dissolution of Component I+II+III Precipitate
[0112] Precipitate 56.0kg of component I+II+III with an aqueous solution containing sodium dihydrogen phosphate at 1.1°C (containing 0.54kg of sodium dihydrogen phosphate, 391.7kg of water for injection) and stir for more than 6 hours until completely dissolved to obtain component I+II +III precipitation solution 448.2kg.
[0113] Step 2: Separation of Component I+III-1 Precipitation
[0114] Use 2.9kg of 1mol / L acetic acid solution to adjust the pH value of the above-mentioned component I+II+III precipitation solution to 5.10, use -15°C refrigerant to cool down to -0.3°C, and then add -20.5°C 95% (v / v) ethanol Solution 28.0kg, and use 0.5mol / L sodium hydroxide solution to adjust the pH value to 5.13, use -15°C refrigerant to adjust the final suspension temperature to -2.9°C, stir at a stirring speed of 82rpm for 2.8 hours, add diatomaceous earth after the reaction is complete 1.9kg and 1.2kg of perlite were stir...
Embodiment 3
[0134] Step 1: Dissolution of Component I+II+III Precipitate
[0135] Precipitate 54.6 kg of component I+II+III with 3.1°C aqueous solution containing sodium dihydrogen phosphate (containing 0.53 kg of sodium dihydrogen phosphate, 382.1 kg of water for injection) and stir for more than 6 hours until completely dissolved to obtain component I+II +III precipitation solution 437.3kg.
[0136] Step 2: Separation of Component I+III-1 Precipitation
[0137] Use 2.9kg of 1mol / L acetic acid solution to adjust the pH value of the above-mentioned component I+II+III precipitation solution to 5.06, use -15°C refrigerant to cool down to -0.2°C, and then add -20.8°C 95% (v / v) ethanol Solution 27.3kg, and adjust the pH value to 5.19 with 0.5mol / L sodium hydroxide solution, adjust the final suspension temperature to -3.0°C with -15°C refrigerant, stir at a stirring speed of 83rpm for 2.1 hours, add diatomaceous earth after the reaction is complete 1.9kg and 1.2kg of perlite, stirred at a st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com