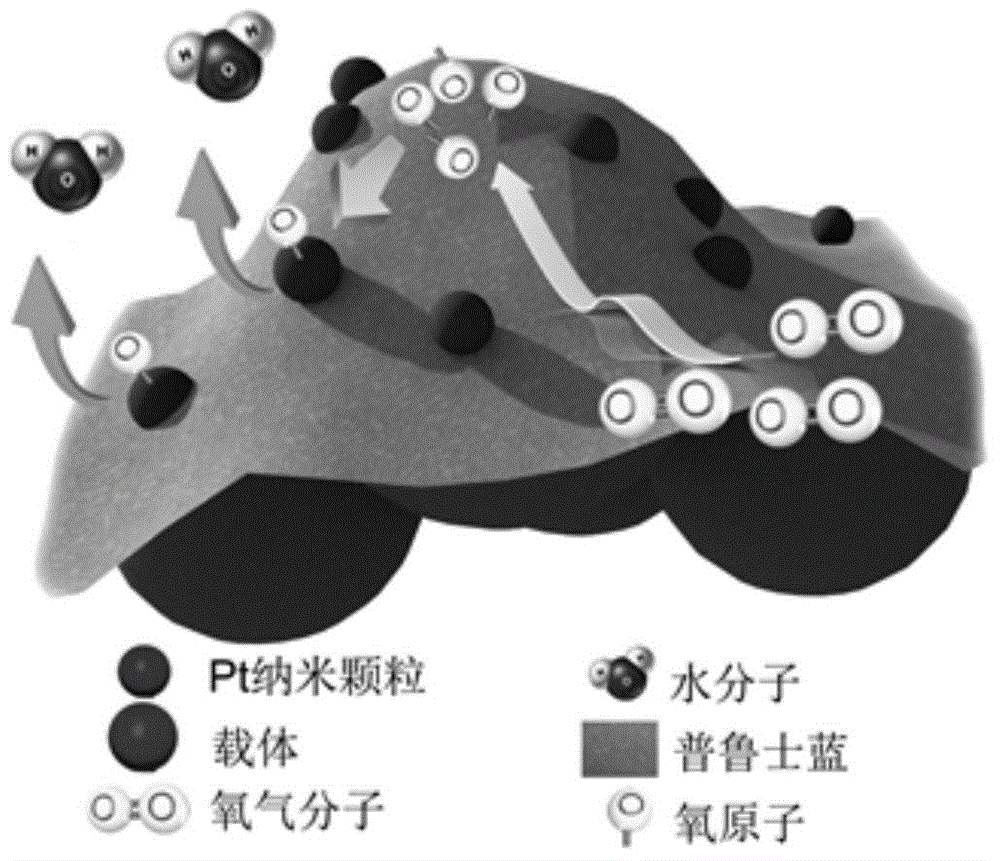
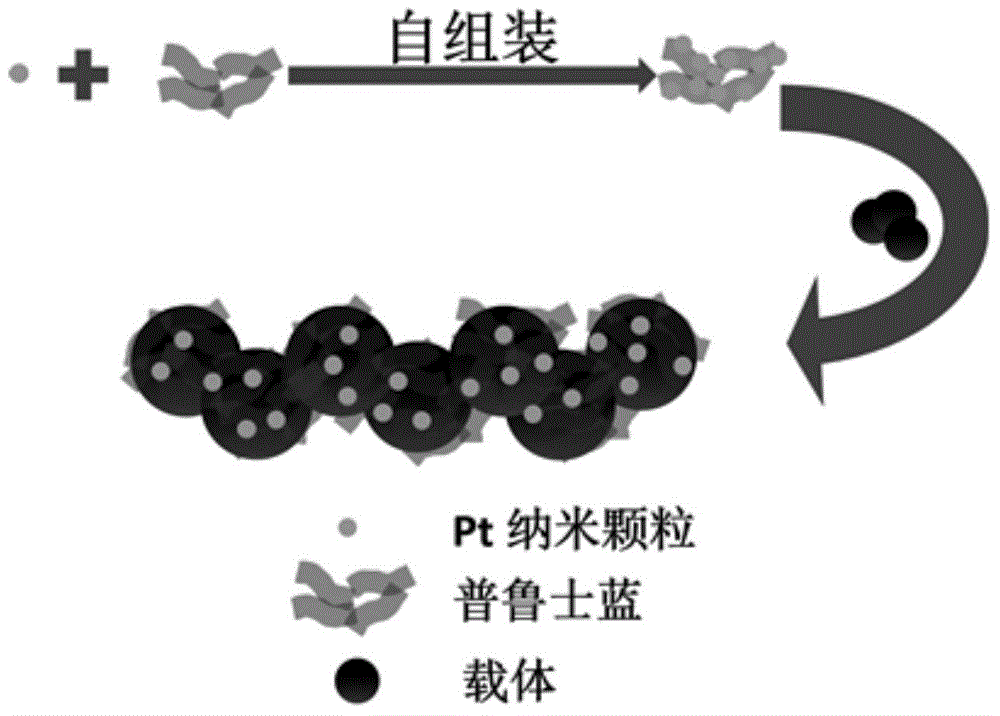
Prussian blue-based high-stability high-activity Pt-based catalyst for fuel cell and preparation method thereof
A Prussian blue, fuel cell technology, applied in chemical instruments and methods, physical/chemical process catalysts, battery electrodes, etc., can solve the problems of cathode catalyst activity and stability, and achieve the goal of improving electrocatalytic activity and increasing stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Get 2.8mL chloroplatinic acid solution (Pt content: 3.8mgPt mL -1 ) was dissolved in 100 mL of water, 150 μL of polydiallyl dimethyl ammonium chloride was added as a stabilizer, and stirred for 30 min. Add strong reducing agent NaBH 4 Solution (20mg NaBH 4 dissolved in 10 mL of water), and continued stirring at room temperature for 2 h to completely reduce the Pt precursor to obtain the Pt nanoparticle colloid.
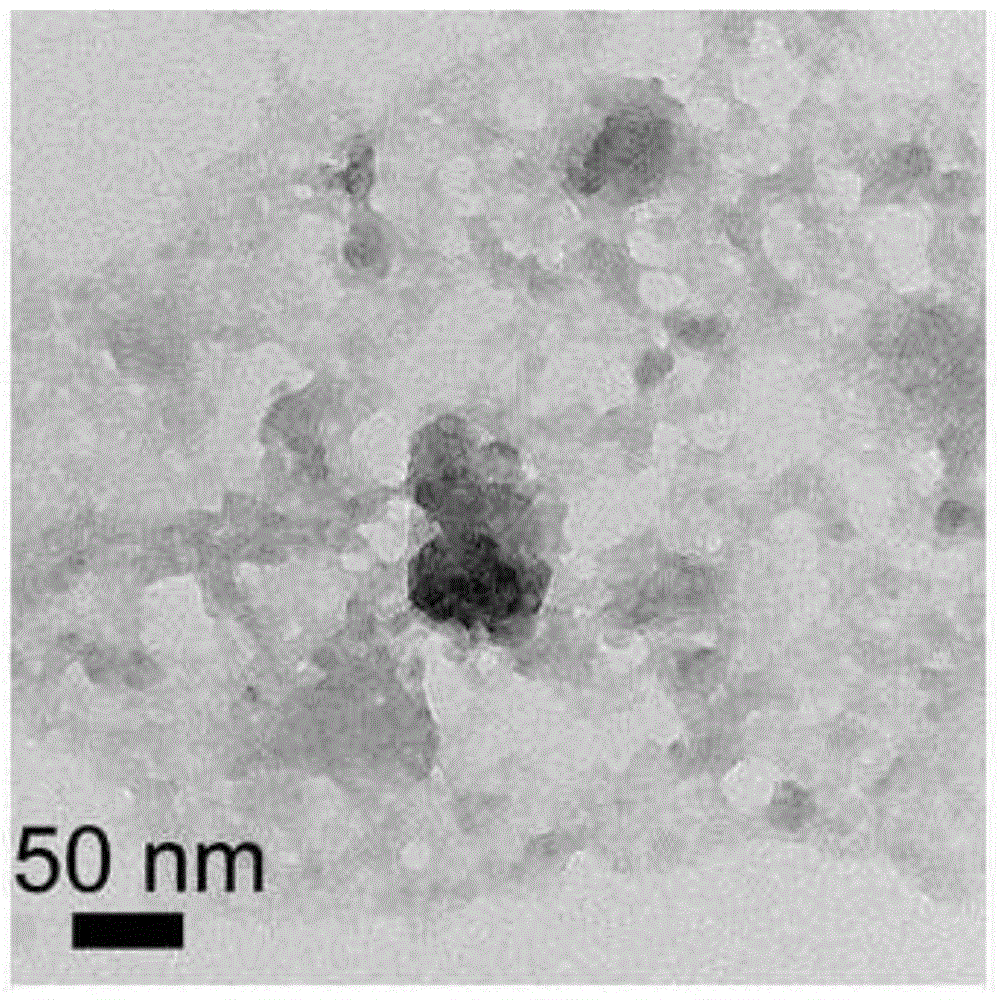
[0039] The Pt nanoparticles are uniform in size, and the particle size distribution is about 2.3nm, such as Figure 12 shown.
Embodiment 2
[0041] Take 4.6mL ferric chloride solution (5mmol L -1 ) was diluted with 100 mL of water. To which was added 1.5mL H 2 o 2 (30wt%) after ultrasonication for 20s, immediately add 3.4mL potassium ferrocyanide solution (5mmol L -1 ), sonicated for 2 hours to obtain PB colloid. according to figure 2 In the preparation process shown, the Pt colloid solution in Example 1 was mixed with the PB colloid for 24 hours to obtain the Pt-PB composite colloid solution, and 35 mg of Vulcan XC-72R was added thereto, fully ultrasonicated for 2 hours, and stirred for 24 hours. In order to deposit the Pt-PB composite colloidal particles on the surface of the carrier, it is necessary to add KNO 3 , the precipitant concentration is 0.5mol L -1 . Stirring is continued for 24 hours to deposit the Pt-PB composite colloidal particles on the surface of the carrier, the catalyst is suction filtered or centrifuged, washed and dried, and the Pt-based composite catalyst based on Prussian blue can b...
Embodiment 3
[0043] Take 2.3mL ferric chloride solution (5mmol L -1 ) was diluted with 100 mL of water. To which was added 1.5mL H 2 o 2 (30wt%) after ultrasonication for 20s, immediately add 1.7mL potassium ferrocyanide solution (5mmol L -1 ), sonicated for 2 hours to obtain PB colloid. according to figure 2 In the preparation process shown, the Pt colloid solution in Example 1 was mixed with the PB colloid for 24 hours to obtain the Pt-PB composite colloid solution, and 37.5 mg of Vulcan XC-72R was added thereto, fully ultrasonicated for 2 hours, and stirred for 24 hours. In order to deposit the Pt-PB composite colloidal particles on the surface of the carrier, it is necessary to add KNO 3 , the precipitant concentration is 0.5mol L -1 . Stirring is continued for 24 hours to deposit the Pt-PB composite colloidal particles on the surface of the carrier, the catalyst is suction filtered or centrifuged, washed and dried, and the Pt-based composite catalyst based on Prussian blue can...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com