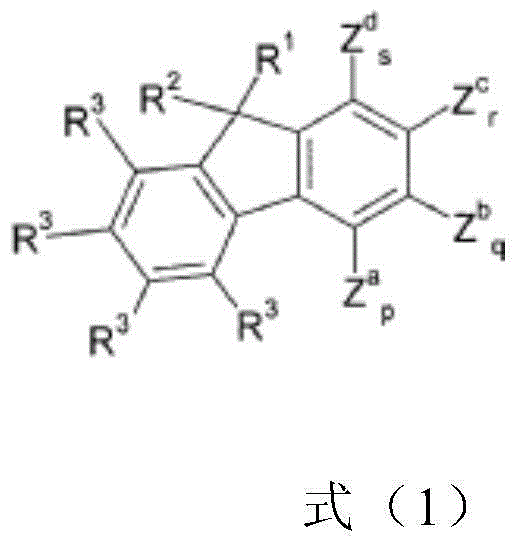
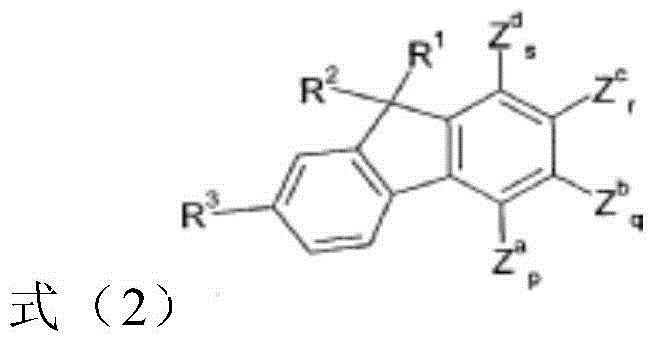
Compounds and organic electroluminescent devices
A technology for electroluminescent devices and compounds, which can be used in electroluminescent light sources, electro-solid devices, preparation of amino compounds, etc., and can solve problems such as high working voltage and poor performance data.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0307] Synthesis of compound biphenyl-2-yl-biphenyl-4-yl-(9-methyl-9-p-tolyl-9H-fluoren-2-yl)-amine (1-1) and compound (1-2) to (1-11)
[0308]
[0309] Among them, Toluene is toluene, and Buchwald-Hartwig coupling is Buchwald-Hartwig coupling.
[0310] 2-Bromo-9-methyl-9-p-tolyl-9H-fluorene
[0311] 40 g (154 mmol) of 2-bromo-9H-fluorene were dissolved in 500 mL of dry THF in a thoroughly heated flask. solution in N 2 Saturated and 15.0 g (170 mmol) of cerium(III) chloride were added. The clear solution was cooled to -10°C and then 121 mL (170 mmol) of 1.4M methylmagnesium bromide solution was added. The reaction mixture was slowly warmed to room temperature, and then washed with NH 4 Quenched with Cl (500 mL). The mixture was then partitioned between acetate and water, the organic phase was washed three times with water, and the 2 SO 4 Dry and rotovap. To the rotovaped solution was added 60 mL of toluene. The precipitate was heated to 50°C and then 27.2 mL of tr...
example 2
[0323] The synthetic compound biphenyl-4-yl-(9,9-dimethyl-9H-fluoren-2-yl)-(9-methyl-9-phenyl-9H-fluoren-4-yl)-amine (2 -1) and compounds (2-2) to (2-8)
[0324]
[0325] Among them, Aminierung means amination
[0326] 4-Bromo-9-methyl-9-phenyl-9H-fluorene
[0327] Dissolve 30 g (94 mmol) of 2,2'-dibromobiphenyl in 200 mL of dry THF in a thoroughly heated flask. The reaction mixture was cooled to -78°C. At this temperature 37.7 mL of a 2.5M solution of n-butyllithium in hexane (94 mmol) were slowly added dropwise (duration: about 1 hour). The precipitate was stirred at -70°C for 1 hour. Then 11.1 mL of acetophenone (94 mmol) was dissolved in 100 ml of THF and added dropwise at -70°C. After the addition was complete, the reaction mixture was slowly warmed to room temperature and washed with NH 4 Cl quenched and then concentrated on a rotary evaporator. To the rotovaped solution was carefully added 300 mL of acetic acid and then 50 mL of fuming hydrochloric acid. The ...
example 3
[0337] Synthetic compound biphenyl-4-yl-biphenyl-2-yl-(7,9-diphenyl-9-p-tolyl-9H-fluorene-2-yl)-amine (3-1) and compound (3 -2) to (3-4)
[0338]
[0339] Among them, Aminierung means amination, Kupplung means coupling, and Toluen means toluene.
[0340] 2-Bromo-7-phenylfluoren-9-one
[0341]21.6 g (178 mmol) of phenylboronic acid, 60 g (178 mmol) of 2,7-dibromo-fluorene were suspended in 800 mL of dimethoxyethane and 265 mL (533 mmol) of 2M sodium carbonate solution. 6.154 g (5 mmol) of tetrakis(triphenylphosphine) were added to the suspension, and the reaction mixture was heated to reflux for 18 hours. After the reaction mixture had cooled, the organic phase was separated, filtered on silica gel, washed 3 times with 100 mL of water and then concentrated to dryness. 38.6 g (85%) of 2-bromo-7-phenylfluoren-9-one were obtained after filtration of the crude product on silica gel through toluene.
[0342] The following brominated compounds were prepared analogously thereto...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com