Improved production process of cefixime tablets
A cefixime and process technology, applied in the field of cefixime tablets and production process, can solve problems such as lack of fluidity, compressibility and lubricity, affect production progress, and easy sticking, etc., to achieve excellent fluidity, Fast release of drugs and the effect of solving the problem of sticking and punching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0011] The following application examples will further illustrate it, and the simple replacements or improvements made by those skilled in the art to the present invention all belong to the technical solutions protected by the present invention.
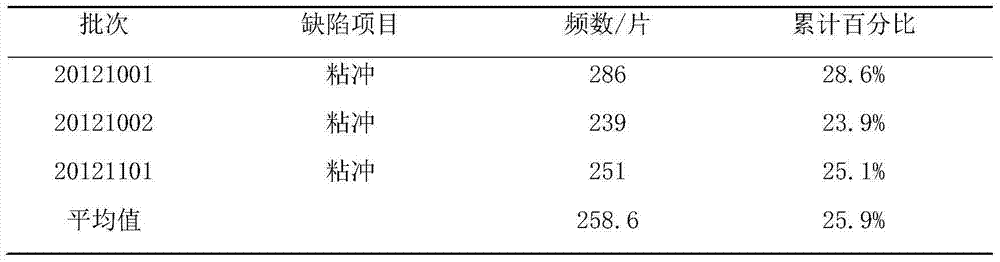
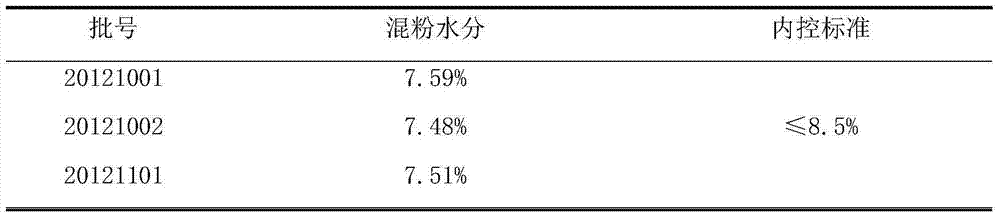
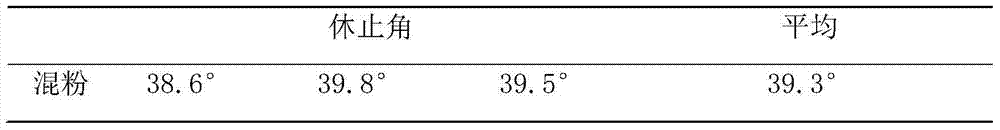
[0012] 1. prepare cefixime tablet, take by weighing the cefixime 100mg of prescription quantity, spray granule lactose 60mg, microcrystalline cellulose 50mg, cross 100 mesh sieves; Take by weighing again the croscarmellose sodium 15mg of prescription quantity, Magnesium stearate 2 mg, passed through a 60-mesh sieve; the above main ingredients and auxiliary materials were placed in a dry granulator, dry granulated, and compressed into tablets to obtain Cefixime Tablets A1. At the same time, the same method and dosage were adopted, and the spray granule lactose was replaced with 200 mesh lactose of the prescription to prepare the Cefixime tablet A2 used in the comparative test.
[0013] 1. First, Cefixime Tablets A2 was used to carry o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com