Application of berberine hydrochloride in preparation of medicine used for preventing and/or treating acute hepatic failure
A technology of berberine hydrochloride and acute liver failure, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1. The protective effect of BBR preventive treatment on the survival rate of mice with acute liver failure
[0056] Methods: Male ICR mice, weighing 22-24 grams, were randomly divided into 3 groups, 7 mice in each group. The three groups were model group, BBR low-dose administration group (5mg / kg) and high-dose administration group (10mg / kg). The administration group was administered by intraperitoneal injection for 3 consecutive days, once a day, and the model group was given the same volume of excipients. One hour after the last administration, animals in each group were intraperitoneally injected with D-GalN 800 mg / kg and LPS 30 μg / kg to establish a model. The survival of mice in each group within 24 h after modeling was recorded.
[0057] Results: The experimental results are shown in Table 1. The survival rate of the mice at 24 hours after modeling was 0 in the model group, while the low-dose and high-dose groups were 28.6% and 71.4% respectively. It can...
Embodiment 2
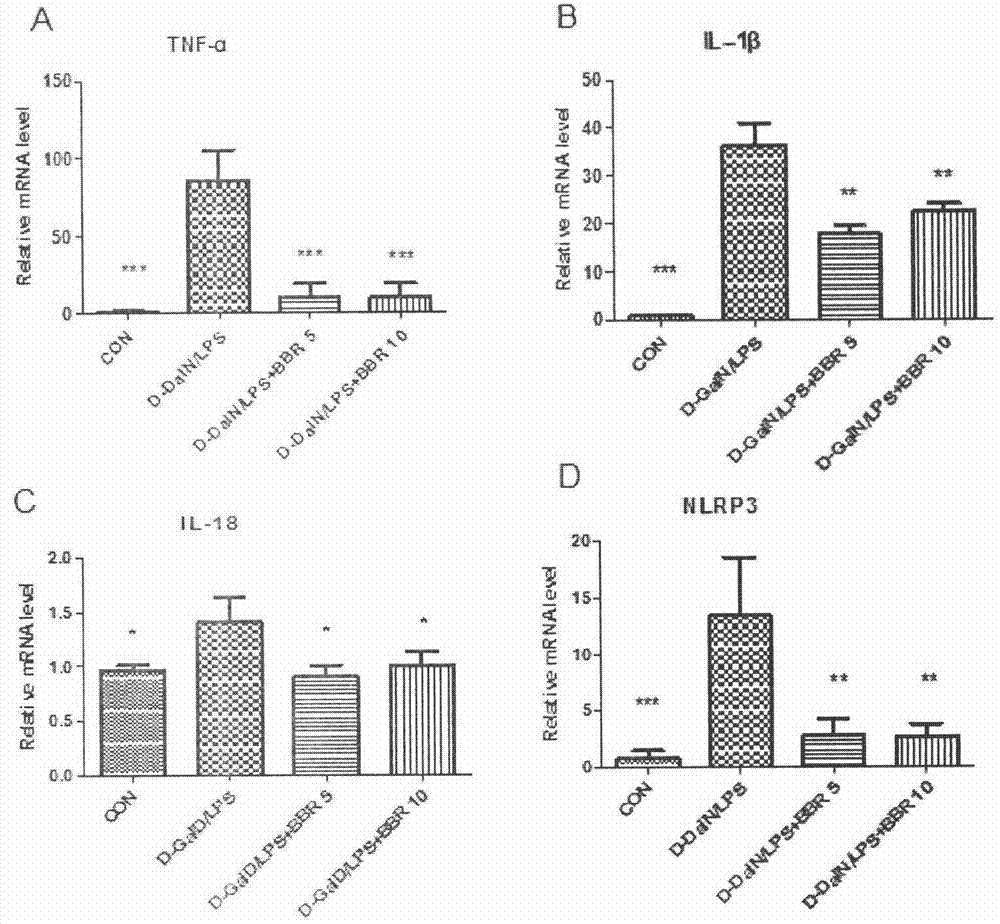
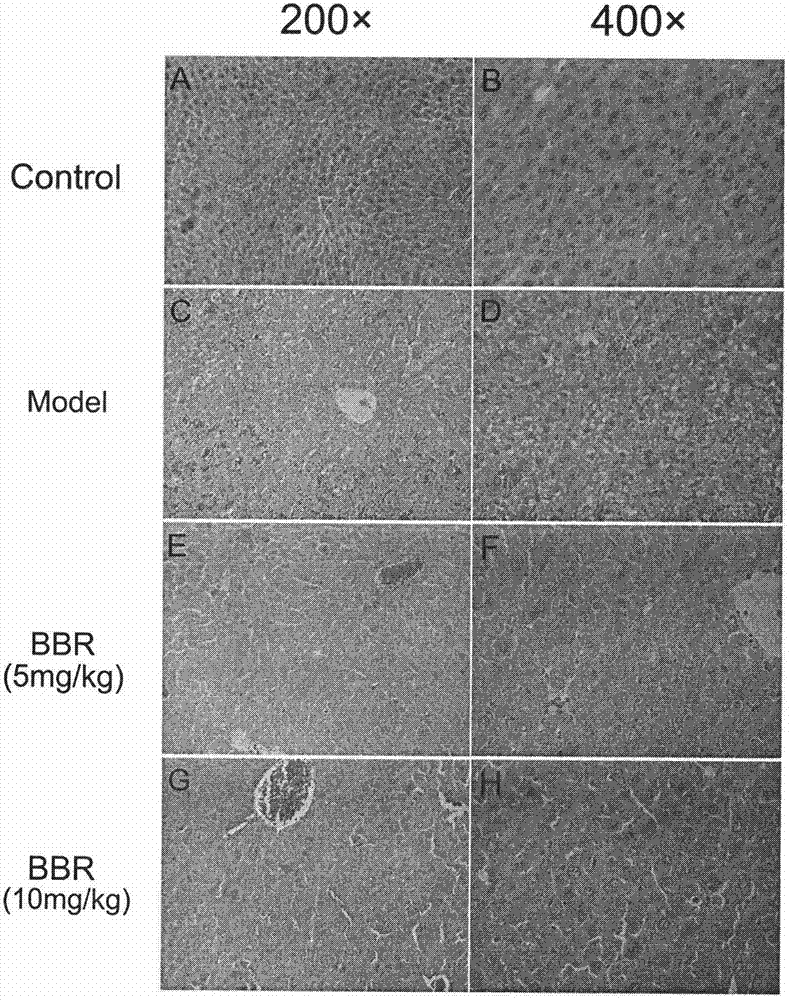
[0060] Example 2. The protective effect of BBR preventive treatment on liver injury and inflammatory injury in mice with acute liver failure
[0061] Methods: Male ICR mice, weighing 22-24 grams, were randomly divided into 4 groups, 10 in each group. The four groups were normal control group, model group, BBR low-dose administration group (5mg / kg) and high-dose administration group (10mg / kg). The administration group was administered by intraperitoneal injection for 3 consecutive days, once a day, and the other groups were given the same volume of excipients. One hour after the last administration, except for the normal control group, animals in other groups were intraperitoneally injected with D-GalN 800 mg / kg and LPS 30 μg / kg to establish models. The mice were sacrificed 2 hours after modeling, and the same liver tissue was taken from different mice. Total RNA was extracted by the Trizol method, and the expression levels of TNF-α, IL-1β and IL-18 mRNA in the liver of the mi...
Embodiment 3
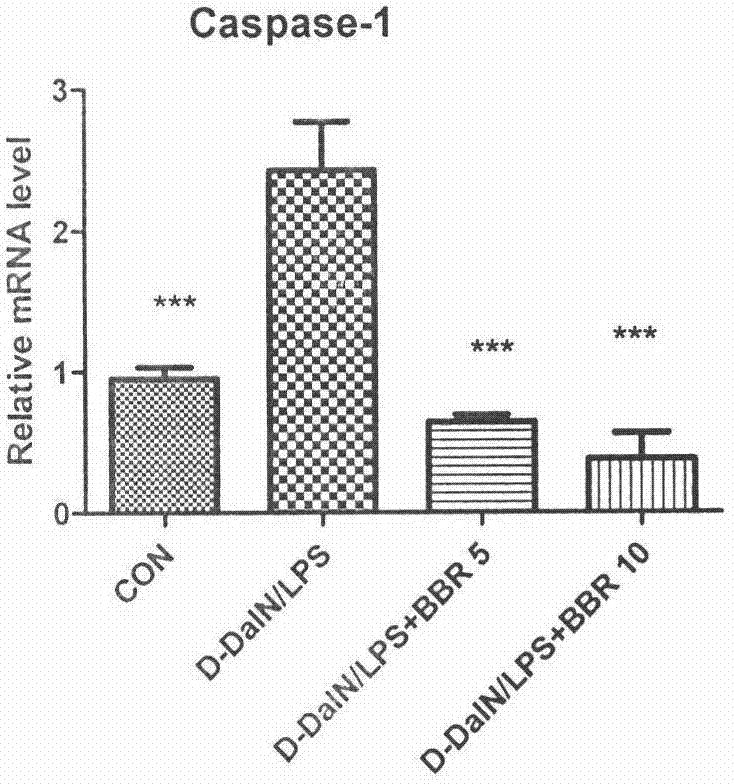
[0066] Example 3. The inhibitory effect of BBR preventive treatment on the expression of Caspase-1 in the liver of mice with acute liver failure
[0067] Methods: Male ICR mice, weighing 22-24 grams, were randomly divided into 4 groups, 10 in each group. The four groups were normal control group, model group, BBR low-dose administration group (5mg / kg) and high-dose administration group (10mg / kg). The administration group was administered by intraperitoneal injection for 3 consecutive days, once a day, and the other groups were given the same volume of excipients. One hour after the last administration, except for the normal control group, animals in other groups were intraperitoneally injected with D-GalN 800 mg / kg and LPS 30 μg / kg to establish models. The mice were sacrificed 2 hours after modeling, and the liver tissues from different mice were collected from the same part, and the total RNA was extracted by Trizol method, and the expression level of Caspase-1 mRNA in mouse...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com