Preparation method of carbon-ferric oxide nanocomposite material
A nanocomposite material, iron oxide nanotechnology, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, chemical/physical processes, etc., can solve the problems of high cost, complex process, and many parameters. , to achieve the effect of tight interface, simple process and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
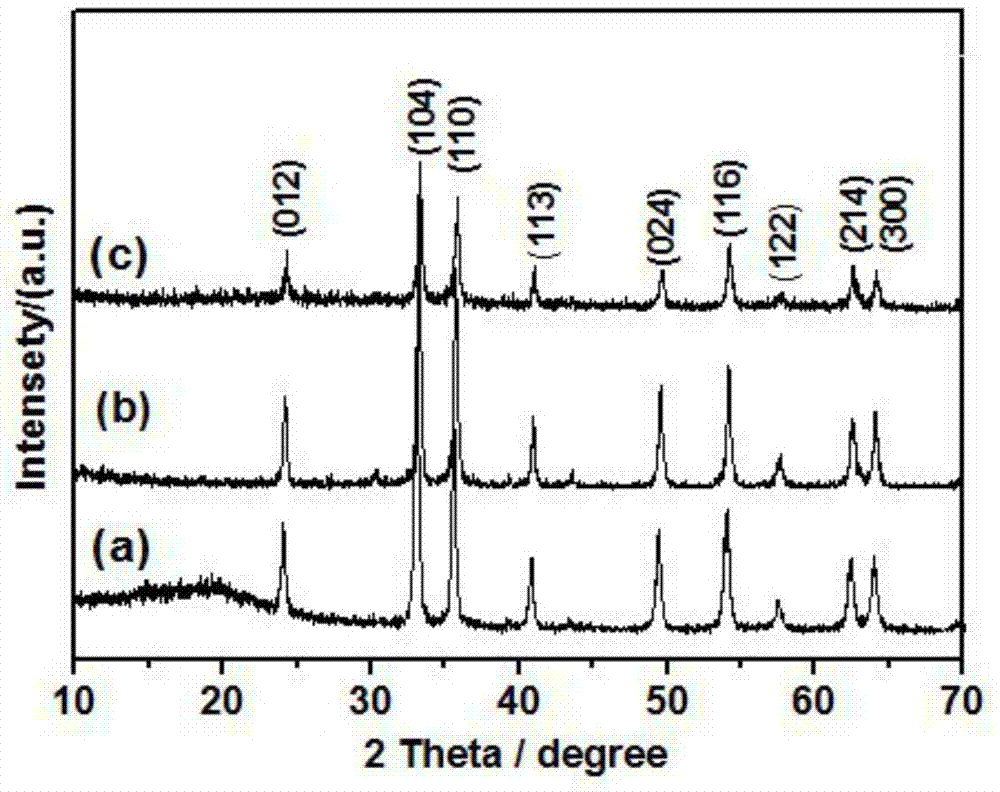
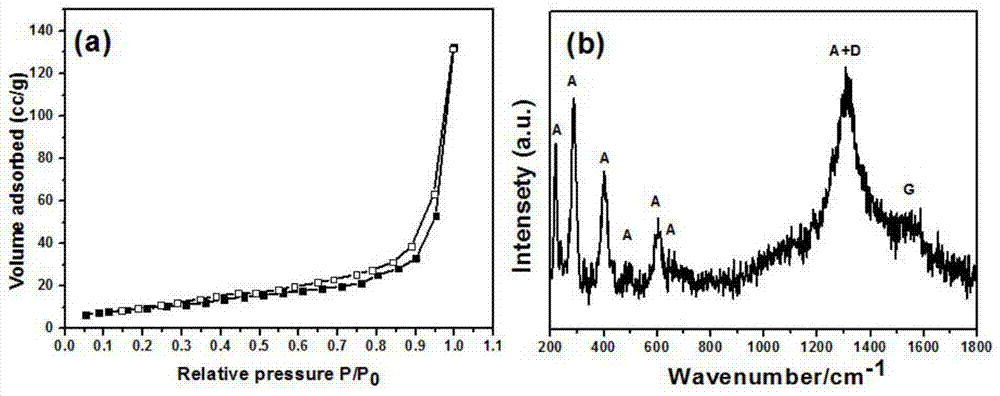
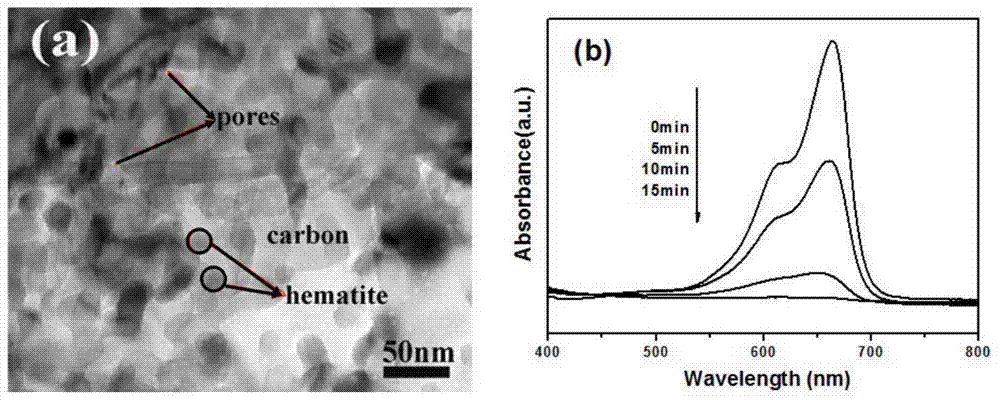
[0018] 0.025mol of ferric nitrate, 0.075mol of amine organic matter, and 0.075mol of carbon source are dissolved in deionized water to prepare a solution, and the solution is placed on a temperature-controllable electric furnace for heating. After the solution undergoes a series of reactions such as volatilization, concentration, and decomposition, a carbon-iron oxide nanocomposite material is obtained. XRD shows that the product has good α-Fe 2 o 3 Structure, Raman results show that there is amorphous carbon in the product, and the nitrogen adsorption-desorption isotherm curve shows that the product has a mesoporous structure. TEM images show that carbon-iron oxide nanocomposites are porous, and α-Fe 2 o 3 Particles of about 30nm, and carbon and α-Fe 2 o 3 The particles are tightly connected. Photocatalytic tests show that carbon-iron oxide nanocomposites have good photocatalytic properties.
Embodiment 2
[0020] 0.025mol of ferric nitrate, 0.0125mol of amine organic matter, and 0.0625mol of carbon source are dissolved in deionized water to prepare a solution, and the solution is placed on a temperature-controllable electric furnace for heating. After the solution undergoes a series of reactions such as volatilization, concentration, and decomposition, a carbon-iron oxide nanocomposite material is obtained. Gained material structure is identical with embodiment 1, α-Fe 2 o 3 Particles of about 50nm, and carbon and α-Fe 2 o 3 The particles are tightly connected. Photocatalytic tests show that carbon-iron oxide nanocomposites have good photocatalytic properties.
Embodiment 3
[0022] 0.025mol of ferric nitrate, 0.0375mol of amine organic matter, and 0.0375mol of carbon source are dissolved in deionized water to prepare a solution, and the solution is placed on a temperature-controllable electric furnace for heating. After the solution undergoes a series of reactions such as volatilization, concentration, and decomposition, a carbon-iron oxide nanocomposite material is obtained. Gained material structure is identical with embodiment 1, α-Fe 2 o 3 Particles of about 45nm, and carbon and α-Fe 2 o 3 The particles are tightly connected. Photocatalytic tests show that carbon-iron oxide nanocomposites have good photocatalytic properties.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com