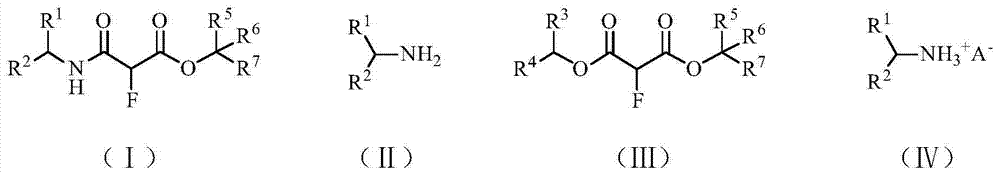
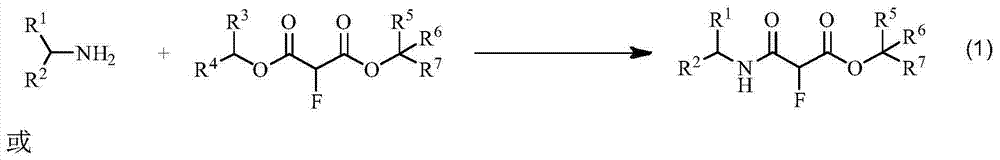
Method for synthesizing alpha-amine formyl fluoroacetate compound
A technology for carbamoyl fluoroacetate and compounds, which is applied in the field of synthesizing α-carbamoyl fluoroacetate compounds, can solve the problems of cumbersome operation, low yield, and long reaction time, and achieve simple preparation and operation simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] In the reactor, 59 mg (1 mmol) of n-propylamine and 1.50 g (10 mmol) of dimethyl fluoromalonate were added, and the reaction was stirred at room temperature. The reaction was monitored by thin-layer chromatography. After 16 hours, the monitoring result showed that n-propylamine had reacted, and the reaction mixture was directly separated by column chromatography to obtain 188 mg of α-(N-propyl)carbamoyl fluoroacetate. The yield was 99%. The product was confirmed by NMR analysis, 1 H NMR (CDCl 3 ,500MHz,δ / ppm):6.43(br,1H),5.22(d,J=49.5Hz,1H),3.71(s,3H),3.32-3.23(m,2H),1.59-1.52(m,2H ), 1.31(t,J=7.3Hz,3H).
Embodiment 2
[0019] Into the reactor, 59 mg (1 mmol) of isopropylamine and 178 mg (1 mmol) of diethyl fluoromalonate were added, and stirred at room temperature for 8 hours. The mixture was directly separated by column chromatography to obtain 78 mg of ethyl α-(N-isopropyl)carbamoyl fluoroacetate in a yield of 41%. The product was confirmed by NMR analysis, 1 H NMR (CDCl 3 ,500MHz,δ / ppm):6.19(br,1H),5.19(d,J=49.0Hz,1H),4.36-4.26(m,2H),4.16-4.06(m,1H),1.32(t,J =7.3Hz, 3H), 1.19(t, J=6.0Hz, 6H).
Embodiment 3
[0021] Into the reactor, 194 mg (2 mmol) of 2-furylmethylamine and 1.78 g (10 mmol) of diethyl fluoromalonate were added, and stirred at room temperature for 16 hours. The reaction mixture was dispersed in 15 mL of ether, filtered, and the filter cake was washed with 5 mL of ether. The filter cake was recrystallized from ethanol to obtain 390 mg of ethyl α-(N-(2-furylmethyl))carbamoyl fluoroacetate, with a yield of 85%. The product was confirmed by NMR analysis, 1 H NMR (CDCl 3 ,500MHz,δ / ppm):7.36-7.35(m,1H);6.73(br,1H),6.32-6.31(m,1H);6.26-6.25(m,1H),5.26(d,J=48.5Hz ,1H),4.55-4.42(m,2H),4.36-4.26(m,2H),1.32(t,J=7.3Hz,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com